Core Practical: Investigating Rate of Reaction (Edexcel GCSE Chemistry): Revision Note
Exam code: 1CH0
Determining the Rate of a Reaction
To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed
The method used for measuring depends on the substances involved
There are a number of ways to measure a reaction rate in the lab; they all depend on some property that changes during the course of the reaction
That property is taken to be proportional to the concentration of the reactant or product, e.g., colour, mass, volume
Some reaction rates can be measured as the reaction proceeds (this generates more data);
faster reactions can be easier to measure when the reaction is over, by averaging a collected measurement over the course of the reaction
Three commonly used techniques are:
measuring mass loss on a balance
measuring the volume of a gas produced
measuring a reaction where there is a colour change at the end of the reaction
Changes in Mass
When a gas is produced in a reaction it usually escapes from the reaction vessel, so the mass decreases
This can be used to measure the rate of reaction
For example, the reaction of calcium carbonate with hydrochloric acid produces CO2
The mass is measured every few seconds and change in mass over time is plotted as the CO2 escapes

Measuring mass changes on a balance
Volumes of Gases
When a gas is produced in a reaction, it can be trapped and its volume measured over time
This can be used to measure the rate of reaction.
For example, the reaction of magnesium with hydrochloric acid produces hydrogen

Measuring changes in gas volume
Measuring concentration changes
Measuring concentration changes during a reaction is not easy; the act of taking a sample and analysing it by titration can affect the rate of reaction (unless the reaction is deliberately stopped- this is called quenching).
Often it is more convenient to ‘stop the clock’ when a specific (visible) point in the reaction is reached
For example when a piece of magnesium dissolves completely in hydrochloric acid
Another common rate experiment is the reaction between sodium thiosulfate and hydrochloric acid which slowly produces a yellow precipitate of sulfur that obscures a cross when viewed through the solution:
Na2S2O3 (aq) + 2HCl (aq) → 2NaCl (aq) + SO2 (g) + H2O (l) + S (s)

The disappearing cross experiment
Calculating rates of reaction
Reactions take place at different rates depending on the identities and conditions
Some are extremely slow e.g. rusting and others are extremely fast e.g. explosives
Rates of reaction can be measured either by how fast a reactant is used up or by how fast the product is made
Rate is concerned with amounts of substances and time and can be calculated using the formula:

A formula triangle for calculating the rate of reaction
In order to provide sufficient data to establish a conclusion several measurements need to be made during the reaction
The product is usually the one that is measured as it is usually easier to measure a product forming than it is a reactant disappearing
The quantity to be measured depends on the reaction and may be in grams for mass or cm3 or dm3 for volume if the product is a gas
The units of the rate of reaction would therefore be g/s or cm3/s or dm3/s
Time is usually in seconds as many reactions studied in the lab are quite quick
If one of the products is a gas which is given off, then the reaction can be performed in an open flask on a balance to measure the loss in mass of reactant
Cotton wool is usually placed in the mouth of the flask which allows gas out but prevents any materials from being ejected from the flask (if the reaction is vigorous)
Calculating Gradients
Often a curved graph is obtained or a graph which starts out as a straight line but then curves to form a horizontal line as the reaction peters out, usually due to one of the reactants running out
The curved section signifies that the relationship between rate and the factor being measured is not directly proportional, so the rate of reaction is different along each point of the curve
For a curve graph a tangent must be drawn to calculate the change in x and y so the rate of reaction at a particular point during the reaction can be calculated
Place a ruler on the point being studied and adjust its position so the space on either side of the point between the ruler and curve are equal:
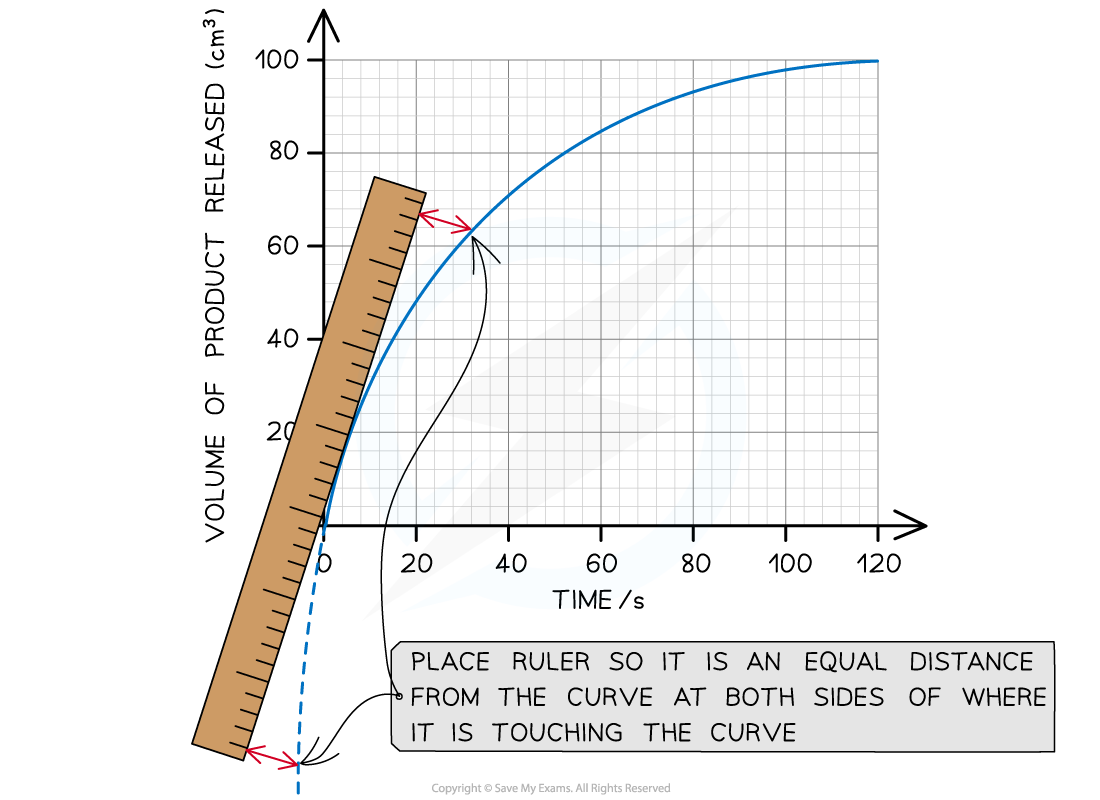
Drawing a tangent to a curve using a ruler
Use the tangent to calculate the rate of reaction as shown below:

Obtaining a tangent on a curve
The gradient at that point is
GRADIENT = ∆ (PRODUCT) ÷ ∆ (TIME)
You can use this formula to calculate the gradient at any particular point in the curve
Examiner Tips and Tricks
When drawing tangents, the line should be extended as far as is convenient for you to perform the calculations. Extending the tangent in this way decreases the amount of uncertainty.
Core Practical: Investigating Rate of Reaction
Part A- Measuring the Production of a Gas
Aim:
To investigate the effect of changing surface area of marble chips in the reaction between marble chips and hydrochloric acid
Materials:
Marble chips, small and large
Hydrochloric acid 1 mol dm-3
Conical flask (100 cm3)
Safety goggles
Gas syringe
Stop clock
Diagram:



Investigating the effect of different size marble chips on the rate of reaction between calcium carbonate and hydrochloric acid
Method:
Add hydrochloric acid into a conical flask
Use a delivery tube to connect this flask to an inverted measuring cylinder
Add marble chips into the conical flask and close the bung
Measure the volume of gas produced in a fixed time using the measuring cylinder
Repeat with different sizes of marble chips
Result:
Increase in the surface area of the marble chip, the rate of reaction will increase
This is because more surface area particles of the marble chips will be exposed to the dilute hydrochloric acid so there will be more frequent and successful collisions, increasing the rate of reaction
Part B- Observing a Colour Change
Aim:
To investigate the effect of changing concentration in the reaction between sodium thiosulfate and hydrochloric acid
Materials:
40 g dm-3 sodium thiosulfate solution
1.0 mol dm-3 dilute hydrochloric acid
Conical flask (100 cm3)
Black cross on paper
White paper or white tile
Stopwatch or timer
Diagram:

Diagram showing the apparatus needed to investigate the effect of concentration on the rate of reaction
Method:
Measure 50 cm3 of sodium thiosulfate solution into a flask
Measure 5 cm3 of dilute hydrochloric acid into a measuring cylinder
Draw a cross on a piece of paper and put it underneath the flask
Add the acid into the flask and immediately start the stopwatch
Look down at the cross from above and stop the stopwatch when the cross can no longer be seen
Repeat using different concentrations of sodium thiosulfate solution (mix different volumes of sodium thiosulfate solution with water to dilute it)
Result:
With an increase in the concentration of a solution, the rate of reaction will increase
This is because there will be more reactant particles in a given volume, allowing more frequent and successful collisions, increasing the rate of reaction
Hazards, risks and precautions

Hazard symbols to show substances that are flammable and toxic
Magnesium is a flammable metal
Dilute hydrochloric acid is not classified as hazardous at the concentrations typically used in this practical, however it may still cause harm to the eyes or the skin
The reaction between sodium thiosulfate and hydrochloric acid produces sulfur dioxide which is toxic if inhaled
Magnesium should be kept away from naked flames, e.g. a Bunsen burner
For dilute hydrochloric acid, avoid contact with the skin and use safety goggles
Take care not to inhale sulfur dioxide gas; asthmatics need to be especially careful and a fume cupboard can be used to avoid exposure

Unlock more, it's free!
Did this page help you?