Core Practical: Electrolysis of Copper(II)Sulfate (Edexcel GCSE Chemistry): Revision Note
Exam code: 1CH0
Did this video help you?
Core Practical: Electrolysis of Copper(II)Sulfate
Part 1- Electrolysis with Passive Electrodes
Aim:
To electrolyse copper(II) sulfate solution using inert(graphite) electrodes
Diagram:

Apparatus for the electrolysis of copper(II)sulfate using passive(inert) electrodes
Method: (Graphite electrodes)
Pour copper sulfate solution into a beaker
Place two graphite rods into the copper sulfate solution. Attach one electrode to the negative terminal of a DC supply, and the other electrode to the positive terminal
Completely fill two small test tubes with copper sulfate solution and position a test tube over each electrode as shown in the diagram
Turn on the power supply and observe what happens at each electrode
Test any gas produced with a glowing splint and a burning splint
Record your observations and the results of your tests
Analysis of results:
Record observations of what happens at each electrode, including the results of the gas tests
Conclusion:
Copper metal is formed at the negative electrode and oxygen gas is formed at the positive electrode
Part 2: Electrolysis with Active Electrodes
Aim:
To electrolyse copper(II) sulfate solution using active( copper) electrodesDiagram:
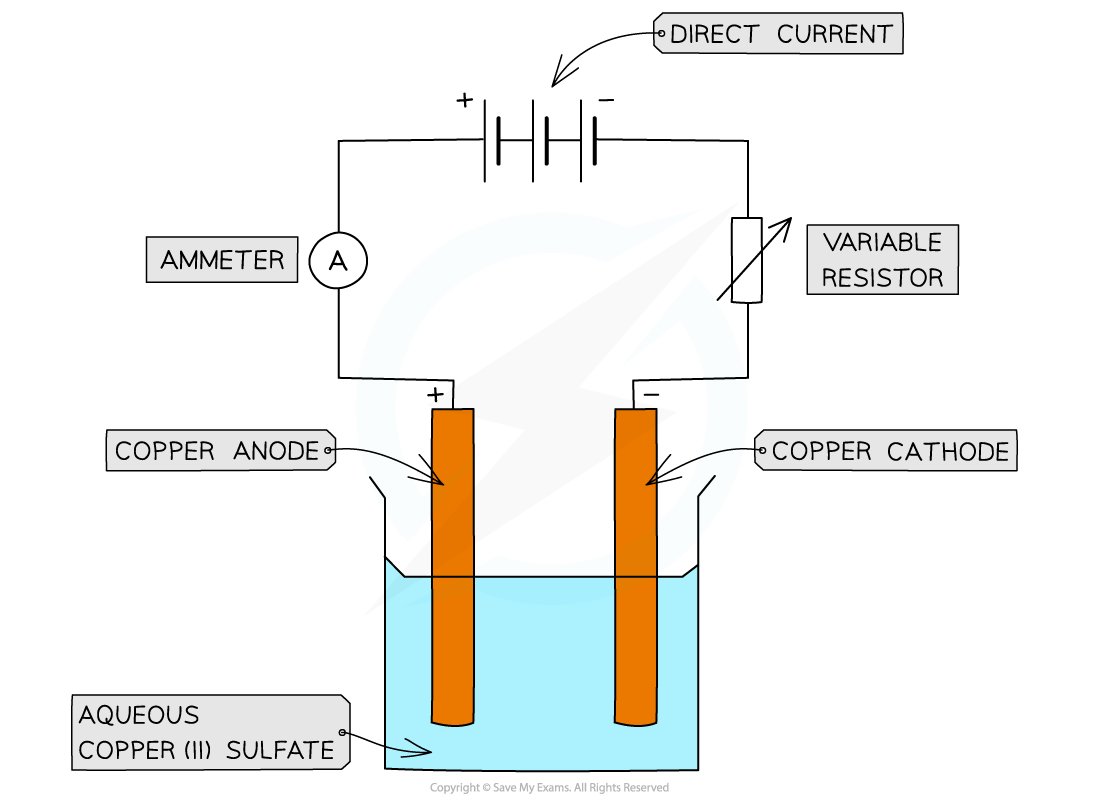
Apparatus for the electrolysis of copper(II)sulfate using active electrodes
Method: (copper electrodes)
Pour copper sulfate solution into a beaker
Measure and record the mass of a piece of copper foil. Attach it to the negative terminal of a DC supply, and dip the copper foil into the copper sulfate solution
Repeat with another piece of copper foil, but this time attach it to the positive terminal
Make sure the electrodes do not touch each other, then turn on the power supply
Adjust the power supply to achieve a constant current and leave for 20 minutes
Remove one of the electrodes and wash it with distilled water, then dip it into propanone
Lift the electrode out and allow all the liquid to evaporate. Do not wipe the electrodes clean. Measure and record the mass of the electrode
Repeat with the other electrode making sure you can identify which electrode is which
Repeat the experiment with fresh electrodes and different currents.
Analysis of results:
Record the currents used and the masses of each electrode in suitable table format
Calculate the change in mass of each electrode
Conclusion:
The cathode increases in mass while the anode decreases
This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the cathode, forming copper atoms
The gain in mass by the negative electrode is the same as the loss in mass by the positive electrode
Therefore the copper deposited on the negative electrode must be the same copper ions that are lost from the positive electrode
That implies that the concentration of the Cu2+ ions in the solution remains constant
Hazards, risks and precautions

Hazard symbols to show substances that are corrosive, harmful to health and flammable
Copper(II) sulfate solution is corrosive and harmful to health as it is a skin irritant and can cause serious eye damage
Propanone, which is often used to clean the electrodes, is flammable
Avoid contact with the skin and use safety goggles when handling copper(II) sulfate solution
Propanone should be kept away from naked flames, e.g. a Bunsen burner
Explaining the Electrolysis of Copper(II)Sulfate
Copper refining
The electrolysis of CuSO4 using graphite rods produces oxygen and copper
By changing the electrodes from graphite to pure and impure copper, the products can be changed at each electrode
Electrolysis can be used to purify metals by separating them from their impurities
In the set-up, the impure metal is always the anode, in this case the impure copper
The cathode is a thin sheet of pure copper
The electrolyte used is an aqueous solution of a soluble salt of the pure metal at the anode, e.g. CuSO4
Copper atoms at the anode lose electrons, go into solution as ions:
Cu ⟶ Cu2+ + 2e
The anode thus becomes thinner due to loss of atoms and the impurities fall to the bottom of the cell as sludge
The copper(II) ions are attracted to the cathode where they gain electrons and form now purified copper atoms
The cathode gradually becomes thicker
Cu2+ + 2e- ⟶ Cu
The anode sludge is a highly valuable material and is further refined as it often contains small quantities of precious metals like silver which are found as impurities in the unrefined copper

Unlock more, it's free!
Did this page help you?