Collision Theory & Activation Energy (AQA GCSE Chemistry) : Revision Note
Did this video help you?
Collision theory
Particle theory states that chemical reactions occur only when the reactant particles collide with sufficient energy to react
The minimum amount of energy needed is called the activation energy
Activation energy is different for each reaction
Collisions can be described as successful or unsuccessful
A successful collision
A successful collision means that the reactant particles colliding have sufficient energy, i.e. greater than or equal to the activation energy
This means that the reactant particles rearrange to form the products
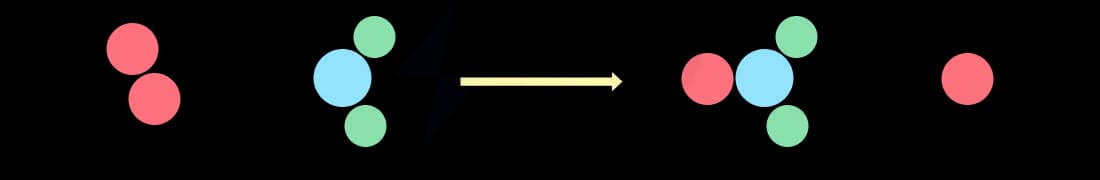
The collision is successful resulting in a rearrangement of atoms to form the products
An unsuccessful collision
An unsuccessful collision means that the reactant particles have insufficient energy, i.e. less than the activation energy
This means that the reactant particles just bounce off each other and remain unchanged

The collision is unsuccessful resulting in no rearrangement of atoms
Increasing the number of successful collisions means that a greater proportion of reactant particles collide to form product molecules
The following all affect the rate of reaction which is dependent on the number of successful collisions per unit time:
The number of particles per unit volume - more particles in a given volume will produce more frequent successful collisions
The frequency of collisions - a greater number of collisions per second will give a greater number of successful collisions per second
The kinetic energy of the particles - greater kinetic energy means a greater proportion of collisions will have an energy that exceeds the activation energy and the more frequent the collisions will be as the particles are moving quicker, therefore, more collisions will be successful
The activation energy - if a reaction has a high activation energy, there will be fewer collisions with an energy that exceeds the activation energy and fewer collisions will be successful
So, the rate of a reaction is dependent on the energy of collisions as well as the number of collisions
Explaining rates
Increasing the number of successful collisions means that a greater proportion of reactant particles collide to form product molecules
We have seen previously that the following factors influence the rate of reaction
Increasing concentration / pressure
Increasing temperature
Increase the surface area of a solid reactant
Use of a catalyst
We can use collision theory to explain why these factors influence the reaction rate:
How increasing concentration affects rate
Increasing the concentration of a solution increases the rate of reaction
Increasing the concentration means that there are more reactant particles in a given volume
This causes more collisions per second
Leading to more frequent and successful collisions per second
Therefore, the rate of reaction increases
If you double the number of particles, you will double the number of collisions per second
The number of collisions is proportional to the number of particles present
Diagram showing the effect of increasing concentration

A higher concentration of particles in (b) means that there are more particles present in the same volume than (a) so the number of collisions and successful collisions between particles increases causing an increased rate of reaction
How increasing pressure affects rate
Increasing the pressure of a gas increases the rate of reaction
Increasing the pressure means that there are the same number of reactant particles in a smaller volume
This causes more collisions per second
Leading to more frequent and successful collisions per second
Therefore, the rate of reaction increases
Diagram showing the effect of increasing pressure

The higher pressure (b) means that there are the same number of particles present in a smaller volume than (a) so the number of collisions and successful collisions between particles increases causing an increased rate of reaction
How increasing temperature affects rate
Increasing the temperature increases the rate of reaction
Increasing the temperature means that the particles have more kinetic energy
This causes more collisions per second
Leading to more frequent and successful collisions per second
Therefore, the rate of reaction increases
The effect of temperature on collisions is not so straightforward as concentration or surface area; a small increase in temperature causes a large increase in rate
For aqueous and gaseous systems, a rough rule of thumb is that for every 10 oC increase in temperature, the rate of reaction approximately doubles
Diagram showing the effect of increasing temperature

An increase in temperature causes an increase in the kinetic energy of the particles. The number of successful collisions increases
How increasing the surface area affects rate
Increasing the surface area increases the rate of reaction
Increasing the surface area means that a greater surface area of particles will be exposed to the other reactant
This causes more collisions per second
Leading to more frequent and successful collisions per second
Therefore, the rate of reaction increases
If you double the surface area, you will double the number of collisions per second
Diagram showing the effect of increasing surface area

An increase in surface area means more collisions per second
Examiner Tips and Tricks
Temperature affects reaction rate by increasing the number of collisions and the energy of the collisions. Of the two factors, the increase in energy is the more important one.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
