Calculating Gradients (AQA GCSE Chemistry) : Revision Note
Calculating gradients
Higher tier only
Often a curved graph is obtained or a graph which starts out as a straight line but then curves to form a horizontal line as the reaction peters out, usually due to one of the reactants running out
The curved section signifies that the relationship between rate and the factor being measured is not directly proportional, so the rate of reaction is different along each point of the curve
For a curve graph a tangent must be drawn to calculate the change in x and y so the rate of reaction at a particular point during the reaction can be calculated
Place a ruler on the point being studied and adjust its position so the space on either side of the point between the ruler and curve are equal:
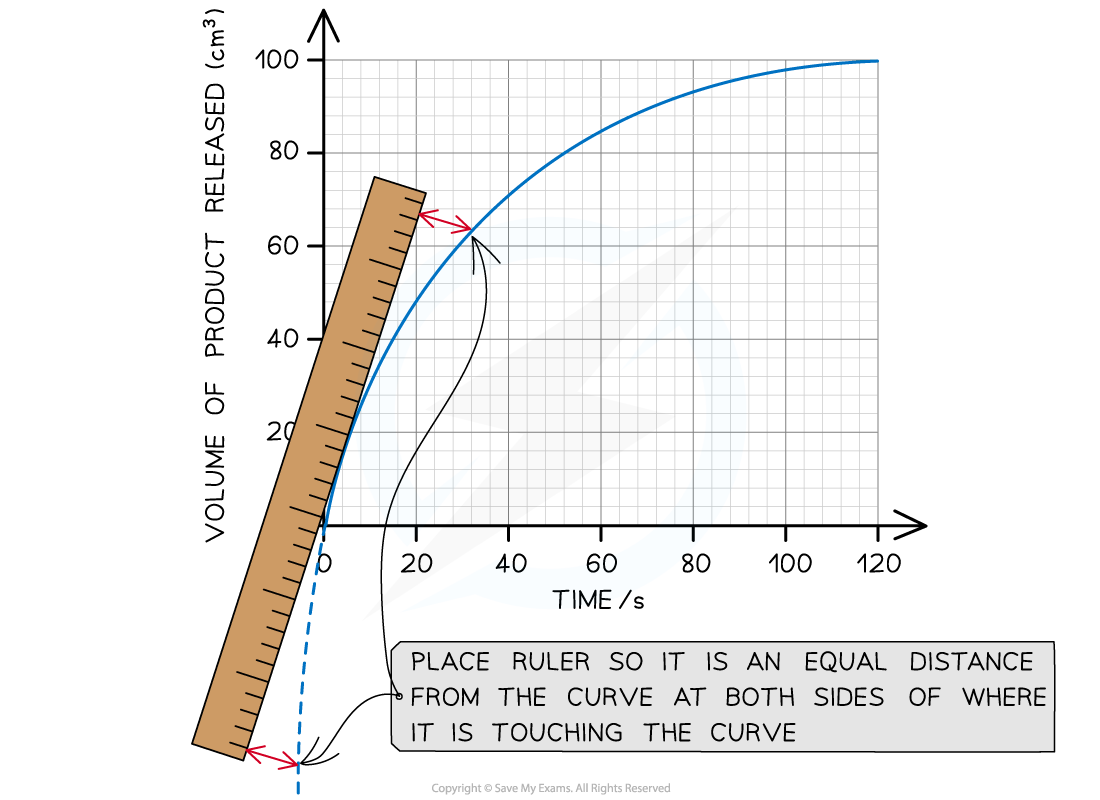
Drawing a tangent to a curve using a ruler
Use the tangent to calculate the rate of reaction as shown below:

Obtaining a tangent on a curve
The gradient at that point is:
gradient =
You can use this formula to calculate the gradient at any particular point in the curve
Examiner Tips and Tricks
When drawing tangents, the line should be extended as far as is convenient for you to perform the calculations. Extending the tangent in this way decreases the amount of uncertainty.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
