Chemical Measurements (AQA GCSE Chemistry) : Revision Note
Uncertainty & error
An error is the difference between a value or quantity obtained in an experiment and an accepted or literature value for an experiment
There are two types of errors in experiments, random errors and systematic errors
Uncertainties are the same as random errors
Uncertainties express the confidence to which the measurement can be taken
Random Errors
When you are reading an instrument and estimate the final digit, there is an equal chance that you may read it slightly too high or slightly too low
This is a random error
Random errors are can be affected by:
How easily the instrument or scale is to read
The person reading the scale poorly
Changes in the environment, for example
fluctuations in the temperature of the lab
air currents in the room
Random errors will pull a result away from an accepted value in either direction (either too high or too low)
Systematic Errors
Systematic errors are errors that occur as a result of a faulty or poorly designed experimental procedure
Systematic errors will always pull the result away from the accepted value in the same direction (always too high or always too low)
For example,
If you forget to zero an electronic balance (using the tare button) the mass weighings will always be higher than they should be
If you don’t read the volume in a burette at eye level, the volumes will always be smaller than they should be due
If you fail to keep a cap on a spirit burner in a calorimetry experiment, the alcohol will evaporate and give you a larger mass loss
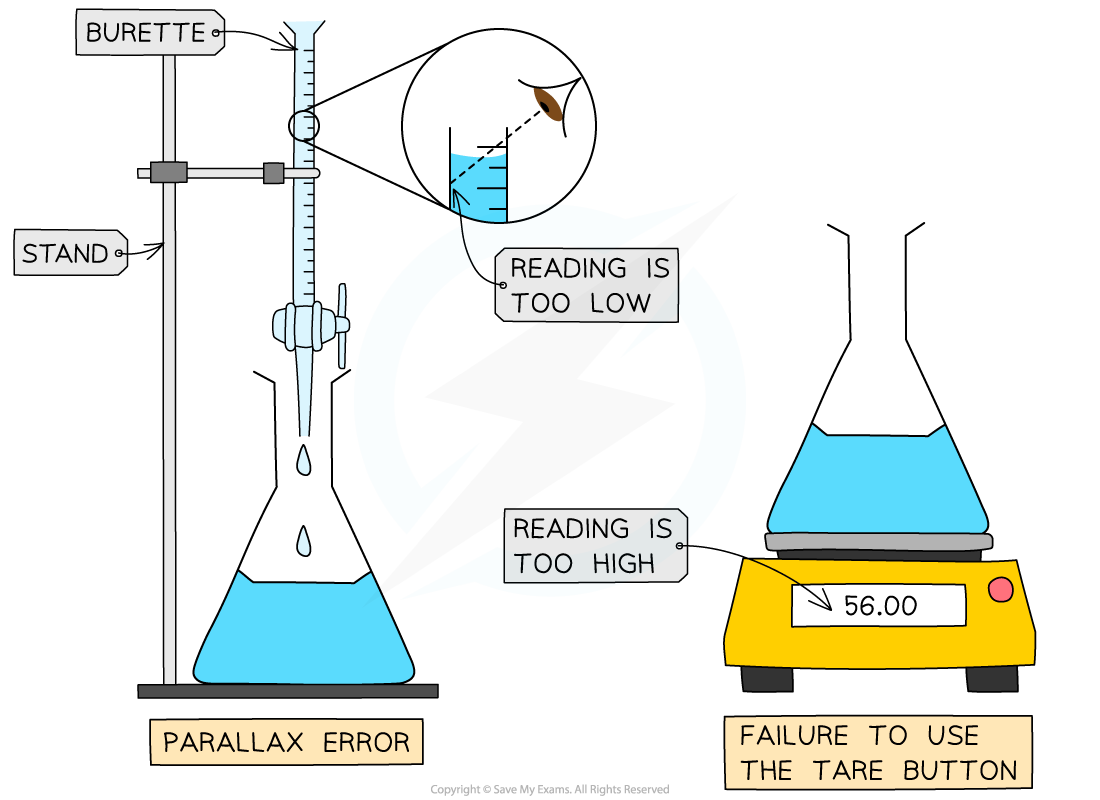
Systematic errors always pull the result away from the accepted value in the same direction: either too high or too low
How to calculate uncertainty
Treatment of uncertainties depends on the type of instrument used
Using analogue instruments
Any instruments that have an analogue scale, the uncertainty is taken as half the smallest division on the scale
For example,
A thermometer that reads to 1oC, the uncertainty would be +0.5 o C
A burette that reads to 0.10 mL, the uncertainty would be +0.05 mL
Using digital instruments
Any instruments that have a digital scale , the uncertainty is taken as the smallest division on the scale
For example,
An electronic balance that reads to 0.01 g, the uncertainty would be +0.01 g
Uncertainty in results
For results that are obtained from a series of repeated experiments, the uncertainty is ± half of the range of results
This can be estimated by:
Calculating the mean average and then determining the deviation of the highest and lowest results from the mean value
An alternative method is to calculate the range of the results and then divide this value by 2
Other uncertainties
Other sources of uncertainty can arise where the judgement of the experimenter is needed to determine a changing property
For example,
Judging the end point of a titration by looking at the colour of the indicator
Controlling a stopwatch in a rate of reaction experiment
Deciding when to extinguish the flame in an experiment
These uncertainties are very difficult to quantify, but they should be commented on as a source of error in an evaluation

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
