Applications of Radioactivity (DP IB Physics) : Revision Note
Applications of Radioactivity
When selecting a radioactive isotope for use in industry, agriculture or medicine, the key factors to consider are
The penetrating power of the decay particle
The half-life of the decay particle
Some key examples which require the use of radioactive isotopes are:
In medicine e.g. radiotherapy, tracers and sterilising equipment
Carbon dating
Uranium-lead dating for ageing rocks
Detecting leaks in underground pipes
Controlling the thickness of materials
Smoke detectors
Carbon Dating
The isotope carbon-14 is commonly used in radioactive dating
It forms as a result of cosmic rays knocking out neutrons from nuclei, which then collide with nitrogen nuclei in the air:
All living organisms absorb carbon-14, but after they die they do not absorb any more
The proportion of carbon-14 is constant in living organisms as carbon is constantly being replaced during the period they are alive
When they die, the activity of carbon-14 in the organic matter starts to fall, with a half-life of around 5730 years
Samples of living material can be tested by comparing the current amount of carbon-14 in them and compared to the initial amount (which is based on the current ratio of carbon-14 to carbon-12), and hence they can be dated
Reliability of Carbon Dating
Carbon dating is a highly reliable method for estimating the ages of samples between 500 and 60 000 years old
This range can be explained by looking at the decay curve of carbon-14:
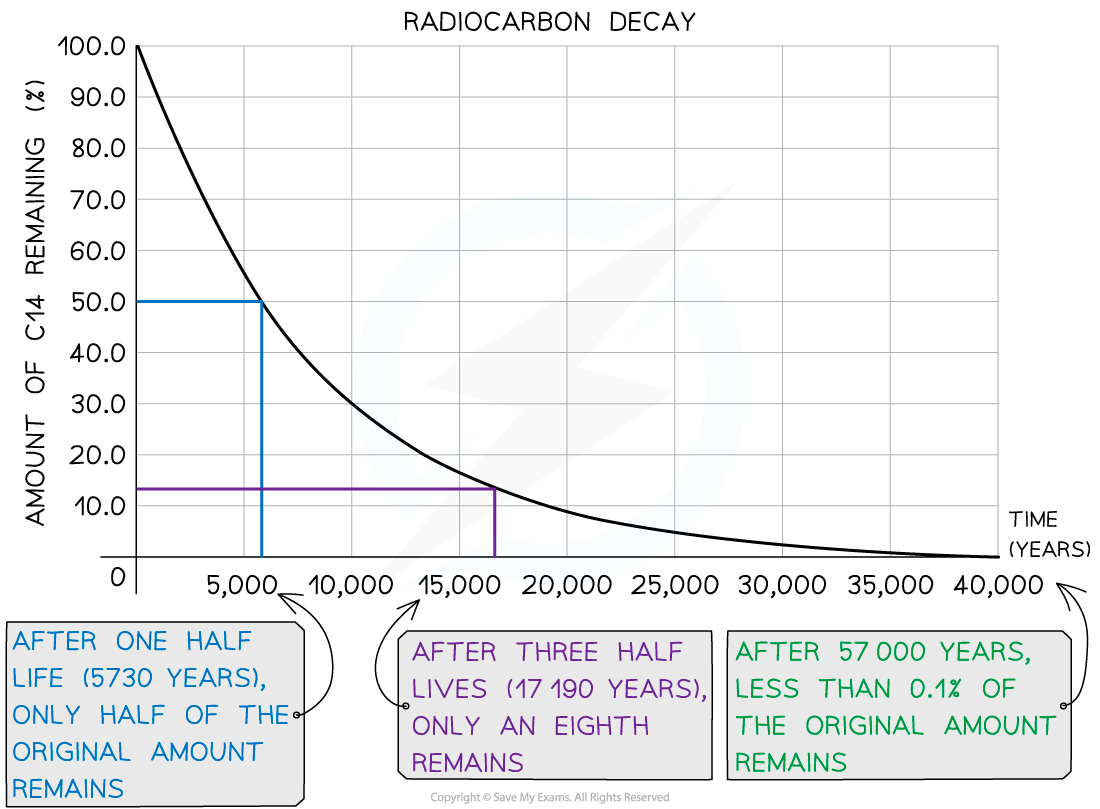
Carbon-14 decay curve used for radiocarbon dating
If the sample is less than 500 years old:
The activity of the sample will be too high to measure small changes accurately
Therefore, the ratio of carbon-14 to carbon-12 will be too high to determine an accurate age
If the sample is more than 60 000 years old:
The activity will be too low to distinguish between changes in the sample and background radiation
Therefore, the ratio of carbon-14 to carbon-12 will be too small to determine an accurate age
Further sources of uncertainty arise with this dating method because:
The level of production of carbon-14 in the atmosphere varies slightly with location
The concentration of carbon-14 in the atmosphere may not have been constant over time
Uranium-Lead Dating
For many years, scientists could not agree on the age of the Earth
Until recently, the Earth was believed to be only millions of years old
Over the last century, radiometric dating methods have enabled scientists to discover the age of the Earth is many billions of years old
The most critical of these methods is uranium-lead dating
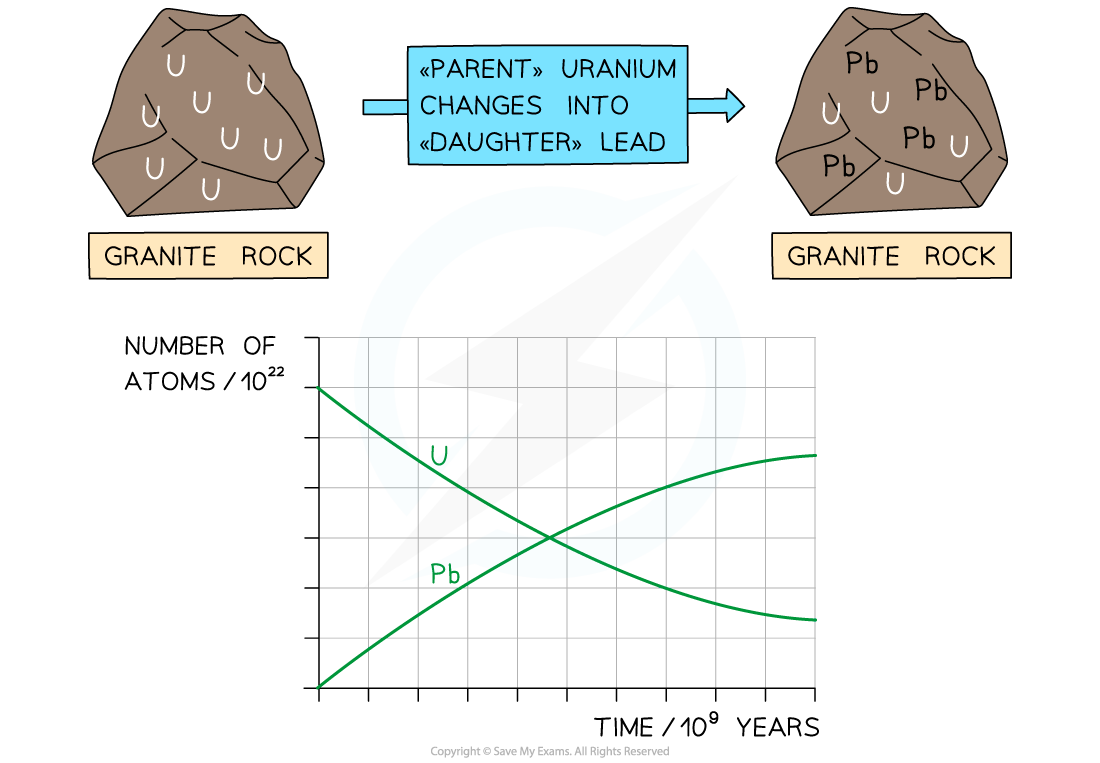
Uranium atoms decay whilst the number of lead atoms increases
Initially, there is only uranium in the rock, but over time, the uranium decays via a decay chain which ends with lead-206, which is a stable isotope
Uranium-238 has a half-life of 4.5 billion years
Over time, the ratio of lead-206 atoms to uranium-238 atoms increases
The ratio of uranium to lead in a sample of rock can then be used to determine its age
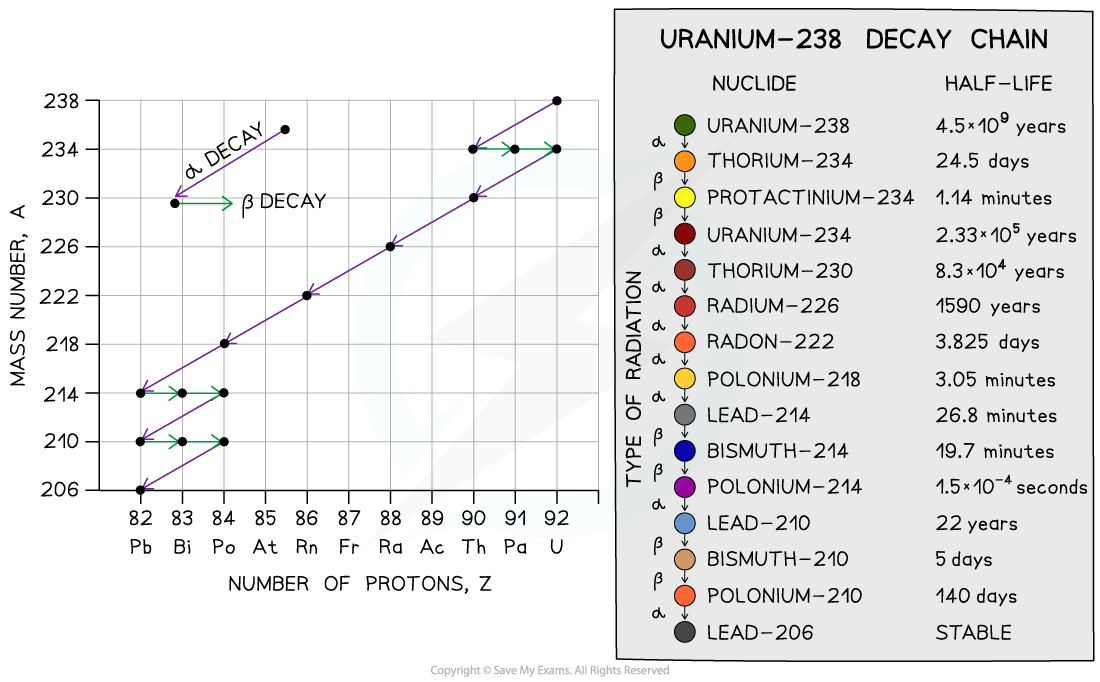
The decay chain of uranium-238 into lead-206 has been crucial for determining an accurate age of the Earth
Detecting Leaks in Underground Pipes
Leaks in underground pipes can be detected by introducing a gamma emitter to the fluid supply in the pipe
By moving a detector along the ground above the pipe, the location of the leak can be identified at the point where an increased count rate is detected
Gamma radiation is required as it is the most penetrating type of radiation
It is the only type of radiation that would be detectable after passing through several metres of ground
Beta radiation could be used if the pipe is not too thick and is near the surface
The half-life of the isotope must be
Long enough for the activity of the source to remain at detectable levels
Short enough that the isotope does not stay present in the supply any longer than required
The isotope sodium-24 is often used in leak detection
It emits both beta and gamma radiation and has a half-life of about 15 hours

The location of a leak in an underground pipe can be found at the point where a detector records a raised count rate compared to the other points along the pipe
Controlling the Thickness of Materials
Beta radiation can be used to determine the thickness of aluminium foil, paper, plastic, and steel
The thickness can be controlled by measuring how much beta radiation passes through the material to a Geiger counter
Beta radiation must be used, because:
Alpha particles would be absorbed by all the materials
Gamma radiation would pass through undetected through the materials
The Geiger counter controls the pressure of the rollers to maintain the correct thickness
A source with a long half-life must be chosen so that it does not need to be replaced often

The pressure of the rollers can be adjusted to control the thickness of the aluminium foil depending on the amount of beta radiation detected
Smoke Detectors
Smoke detectors contain a small amount of americium-241, an alpha emitter
Within the detector, alpha particles are emitted and cause the ionisation of nitrogen and oxygen molecules in the air
These ionised molecules enable the air to conduct electricity by allowing a small current can flow
If smoke enters the alarm, it absorbs the alpha particles, hence reducing the current which causes the alarm to sound
Americium-241 has a half-life of 460 years, so throughout the lifetime of a smoke detector, the activity of the source will not decrease significantly and it will not have to be replaced

The operation of a smoke detector
Worked Example
Below are listed four radionuclides, together with the type of radiation they emit
| radionuclide | type of radiation emitted |
A | americium-241 | alpha (α) |
B | strontium-90 | beta-minus (β–) |
C | cobalt-60 | beta-minus (β–) and gamma (γ) |
D | fluorine-18 | beta-plus (β+) |
Select the most suitable radionuclide in the following applications
(a) Sterilising hospital equipment sealed inside plastic bags
(b) Discharging static electricity that has built up in the manufacture of polyethene
(c) Monitoring the thickness of a thin metal being produced in a factory
(d) A smoke detector
(a) Answer: C
Alpha and low energy beta radiation would most likely be absorbed by the bag
Therefore, gamma radiation, or very high energy beta particles, would be needed to penetrate the bag
This would be best suited to Cobalt-60
(b) Answer: D
Static electricity is an imbalance of electric charges on the surface of the polythene and is generally composed of negatively charged electrons
In order to get rid of the static charge, it will need to be neutralised
Beta-plus particles, or positrons, are the antimatter counterpart of the electron, and hence, are oppositely charged
When the positrons are directed at the surface of the polythene, the electrons will be attracted to them and become neutralised as the particles annihilate as they collide
Therefore, the beta-plus emitter, Fluorine-18, would be best suited to this job
(c) Answer: B
Alpha particles would not be suitable for measuring the thickness of metal as they can be stopped by a thin sheet of paper
Gamma rays are the most penetrating of the radiations and hence would not be suitable where thickness monitoring is up to a few millimetres as they would all pass through
Beta particles are ideally suited as they have enough energy to pass through thin sheets of metal and any changes in thickness would be easily detected
Therefore, the beta-minus emitter Strontium-90 would be the most suitable isotope
(d) Answer: A
Since smoke detectors are present inside homes and other buildings, they must pose no hazard to residents
This means the smoke detector must contain a very small amount of the radioactive material
Also, the radiation should not be too penetrating and should only be able to travel a few centimetres
Therefore, an alpha source should be selected – this means Americium-241 would be the most suitable isotope
Radiation in Medicine
Radionuclides are widely used in medical applications, such as
Radiotherapy
Radioactive tracers
Sterilising equipment
Radiotherapy
Gamma radiation can be used to destroy cancerous tumours
The gamma rays are concentrated on the tumour to protect the surrounding tissue
Less penetrating beta radiation can be used to treat skin cancer by direct application to the affected area


A radiotherapy machine. Powerful radiation is directed at the tumour and lead sheets can be used to prevent healthy tissue from being damaged
Radioactive Tracers
Radioisotopes can be used as ‘tracers’ to monitor the processes occurring in different parts of the body
Radioactive tracers with a short half-life are preferred because:
Initially, the activity is very high, so only a small sample needed
The shorter the half-life, the faster the isotope decays
Isotopes with a shorter half-life pose a much lower risk to the patient
The medical test doesn‘t last long so a half-life of a few hours is enough

A radioactive tracer must be injected into the patient in order to take PET scan images of brain activity
One example is Iodine-131
This isotope is known to be specifically taken up by the thyroid gland making it useful for monitoring and treating thyroid conditions
It emits beta particles which means it will stay concentrated on the thyroid area and nowhere else in the body
It has a short half-life of 8 days meaning it will not be around too long to cause prolonged exposure
Another isotope commonly used as a tracer is Technetium-99m
It is a gamma emitter with an energy of about 140 keV which is ideal for detection
It has a half-life of 6 hours so it is ideal for use as a tracer, but will not remain active for too long and can be tolerated by the body
Gamma radiation is ideal as it is the most penetrating so it can be detected outside the body
Also, gamma is the weakest ioniser and causes minimal damage
As well as this, technetium-99m may be prepared easily at the hospital when required making it a cost-effective treatment
Sterilising Medical Equipment
Gamma radiation is widely used to sterilise medical equipment
Gamma is most suited to this because:
It is the most penetrating out of all the types of radiation
It is penetrating enough to irradiate all sides of the instruments
Instruments can be sterilised without removing the packaging
The general public might be worried that using gamma radiation in this way might cause the equipment itself to become radioactive, however, this is not the case because:
In order for a substance to become radioactive, the nuclei have to be affected
Ionising radiation only affects the outer electrons and not the nucleus
The radioactive material is kept securely sealed away from the packaged equipment so there is no chance of contamination

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
