Alpha, Beta & Gamma Particles (DP IB Physics): Revision Note
Alpha, Beta & Gamma Decay
Some isotopes of elements are unstable
This can happen when a nucleus has an imbalance of protons and neutrons or too much energy
To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay
The three main types of radioactive particle or radiation are:
Alpha particles
Beta particles
Gamma radiation
Alpha Particles
An alpha (α) particle is a high-energy helium nucleus
It contains 2 protons and 2 neutrons
It has a mass of 4u and a charge of +2e
The nuclear notation for an alpha particle is:

Nuclear notation for an alpha particle (a helium nucleus)
Alpha particles are usually emitted by large, unstable nuclei with too many nucleons (protons and neutrons)
When an unstable nucleus decays, its composition changes
When an alpha particle is emitted from a nucleus:
The nucleus loses 2 protons: proton number decreases by 2
The nucleus loses 4 nucleons: nucleon number decreases by 4
As there is a change in proton number, the parent nucleus is a different element to the daughter nucleus
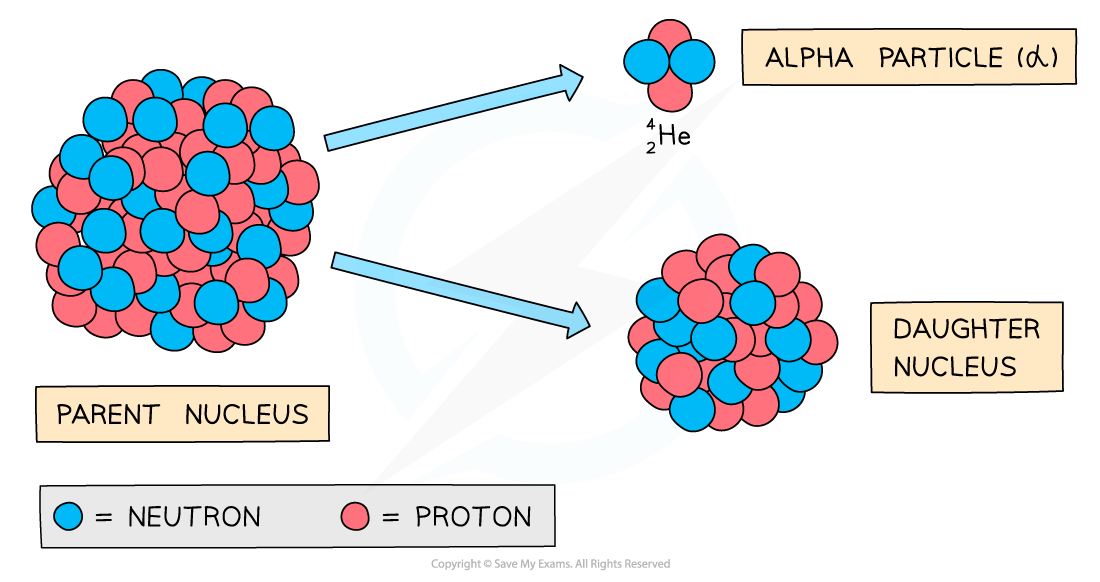
During alpha decay, a parent nucleus becomes a daughter nucleus by emitting an alpha particle (helium nucleus)
Beta-Minus Decay
A beta-minus (β−) particle is a high-energy electron
It has a mass of 0.0005u and a charge of −1e
The nuclear notation for a beta-minus particle is:

Beta-minus particles are usually emitted by unstable nuclei with too many neutrons
Beta-minus decay is when a neutron turns into a proton and emits an electron and an anti-electron neutrino
Electrons have a proton number of −1, so overall:
The proton number increases by 1
The nucleon number remains the same
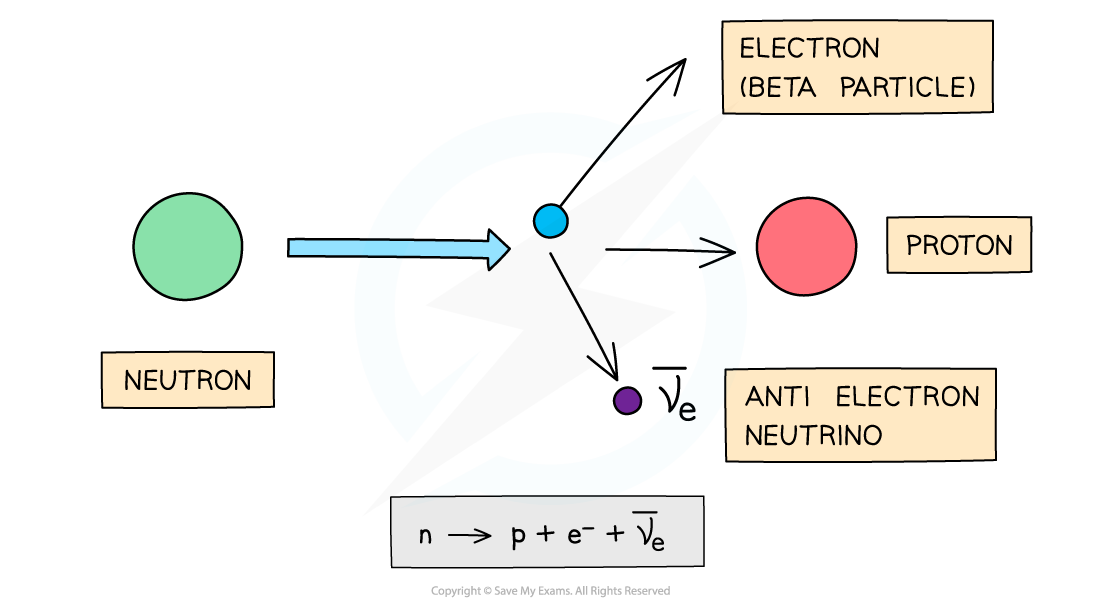
Beta-minus decay often happens in unstable nuclei that have too many neutrons. The nucleon number stays the same, but the proton number increases by one
Beta-Plus Decay
A beta-plus (β+) particle is a high-energy positron
It is the antimatter particle of the electron
It has a mass of 0.0005u and a charge of +1e
The nuclear notation for a beta-minus particle is:

Beta-plus particles are usually emitted by unstable nuclei with too many protons
Beta-plus decay is when a proton turns into a neutron and emits a positron and an electron neutrino
Positrons have a proton number of +1, so overall:
The proton number decreases by 1
The nucleon number remains the same
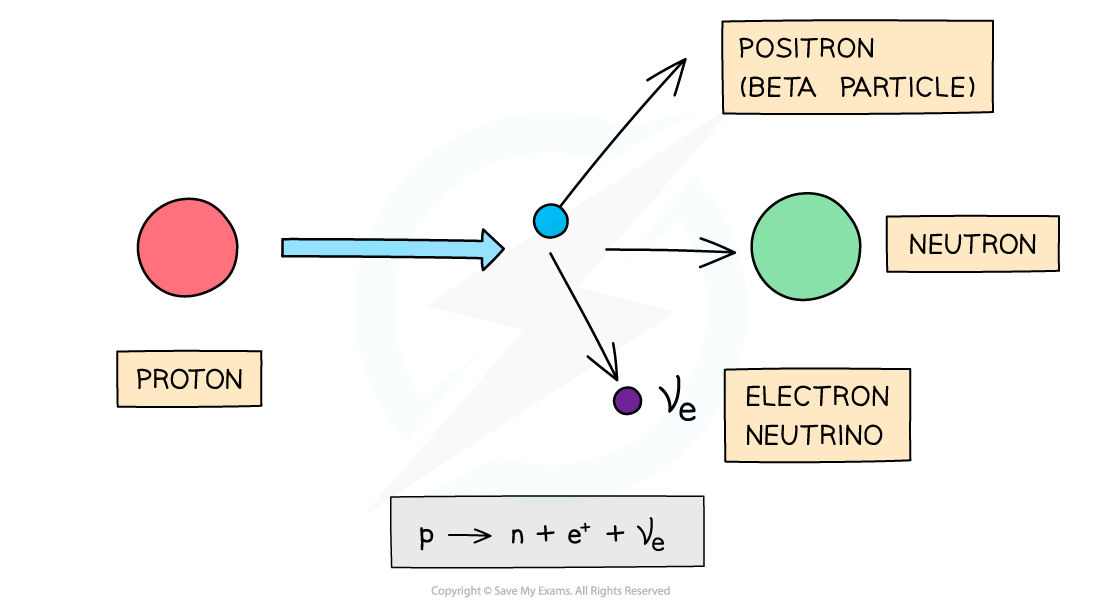
Beta-plus decay often happens in unstable nuclei that have too many protons. The nucleon number stays the same, but the proton number decreases by one
Gamma Radiation
Gamma (γ) rays are a type of high-energy electromagnetic radiation
They are emitted by nuclei that need to lose some energy
The nuclear notation for gamma radiation is:

Nuclear notation for gamma rays
Gamma particles are photons, so they have a proton number of 0, so overall:
The proton number remains the same
The nucleon number remains the same
Worked Example
The radioactive nucleus undergoes alpha decay into a daughter nucleus Po.

(a) Which letter in the diagram represents the daughter product?
(b) What is the nucleon number and proton number of Po?
(a) Answer: C
The number of neutrons in
is 222 − 86 = 136
In alpha decay, the parent nucleus loses a helium nucleus (2 protons, 2 neutrons)
Proton number: 86 decreases to 84
Neutron number: 136 decreases to 134

Therefore, the correct answer is C
(b)
The equation for alpha decay is as follows:

Hence the daughter nucleus Po has
Nucleon number = 222 − 4 = 218
Proton number = 86 − 2 = 84
Worked Example
A radioactive substance with a nucleon number of 212 and a proton number of 82 decays by β-plus emission into a daughter product which further decays by β-plus emission into a granddaughter product.

Which letter in the diagram represents the granddaughter product?
Answer: A
The number of neutrons in the parent nucleus is 212 − 82 = 130
In beta-plus decay, a proton turns into a neutron
Proton number: 82 decreases to 80
Neutron number: 130 increases to 132

Therefore, the correct answer is A
Examiner Tips and Tricks
Remember to avoid the common mistake of confusing the number of neutrons with the nucleon number. In alpha decay, the nucleon (protons and neutrons) number decreases by 4 but the number of neutrons only decreases by 2.
To remember which type of beta emission occurs, try to think of beta ‘plus’ as the ‘proton’ that turns into the neutron (plus an electron neutrino)
Properties of Alpha, Beta & Gamma
Alpha, beta and gamma radiation can be characterised by
Ionising ability - a measure of the amount of ionisation caused when nuclear radiation passes through a material
Penetrating power - a measure of the distance nuclear radiation will travel before losing all its energy
The greater the ionising ability of a type of radiation, the lower its penetrating power, and vice versa
Ionising ability
If any type of radiation collides with an atom, it can knock out electrons, ionising the atom
This can cause chemical changes in materials and damage to living cells
The ionising ability of radiation can be quantified by the number of ion pairs it produces per cm of air
Highly ionising radiation may produce 104 ion pairs per cm of air
Weakly ionising radiation may produce 1 ion pair per cm of air
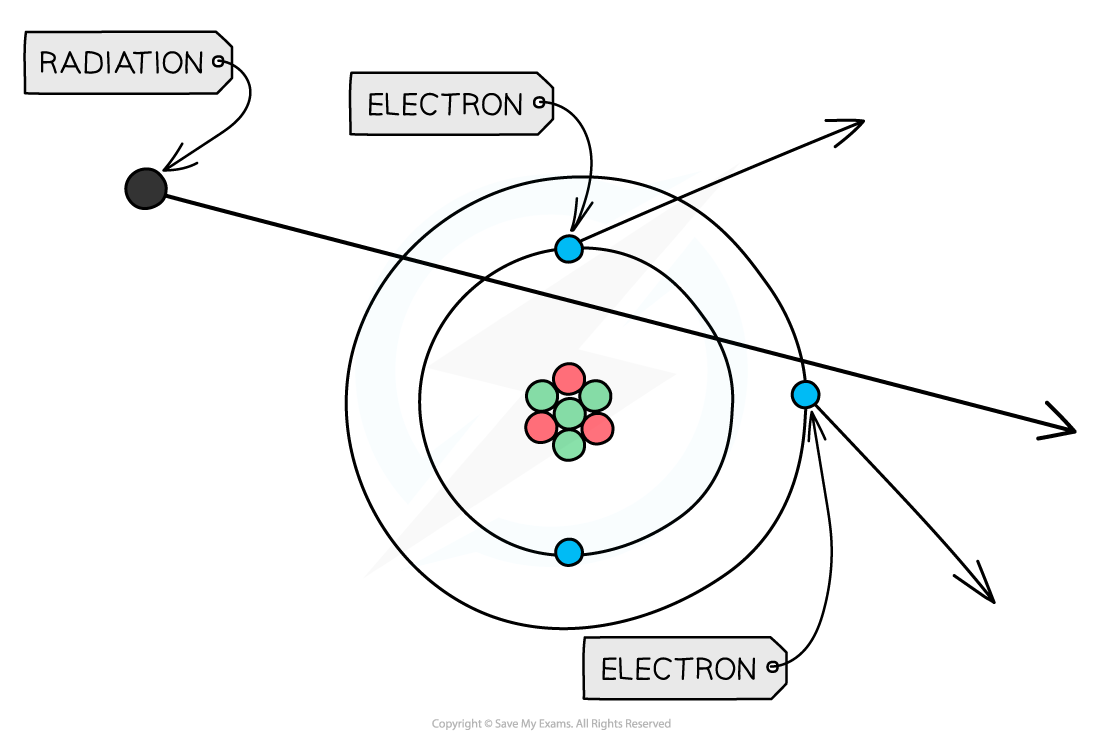
When radiation passes close to atoms, it can knock out electrons, ionising the atom
Penetrating power
The distance radiation can travel before losing most, or all, of its energy, is described by its penetrating power
The lower the penetrating power of a type of radiation, the shorter its range in air
Highly ionising radiation has a low penetrating power
Weakly ionising radiation has a high penetrating power
Deflection in Electric and Magnetic Fields
When a charged particle enters an electric field it will undergo a deflection
Alpha particles are deflected towards the negative plate
Beta particles are deflected towards the positive plate
Gamma radiation is not deflected and travels straight through between the plates

Alpha and beta particles are deflected by an electric field whereas gamma rays are not
When a charged particle moves in a magnetic field, it will also undergo a deflection
Faster-moving particles move in larger circular paths according to the equation:
The larger the circular path, the greater the deflection
The amount of deflection of a particle depends on:
The speed of the particle,
The mass of the particle,
The charge on the particle,
Comparing Alpha, Beta & Gamma
The ionising abilities and penetrating powers of alpha, beta and gamma can be investigated by
Measuring the count rate of a radioactive source using a Geiger counter
Placing different materials between the source and the detector
Measuring the count rate again to see if the material causes a significant reduction
Alpha particles can be stopped by a single sheet of paper
Beta particles can be stopped by a few millimetres of aluminium foil
The intensity of gamma radiation can be reduced by several metres of concrete or several centimetres of lead

Alpha particles are highly ionising and easily absorbed by atoms whereas gamma radiation is highly penetrating and requires very thick lead to reduce its intensity
The properties of the different types of radiation are summarised in the table below:
Comparison of alpha, beta and gamma radiation

Properties of Alpha Radiation
Alpha is the most ionising type of radiation
This is due to it having the highest charge of +2e
This means it produces the greatest number of ion pairs per cm in air
This also means it can do more damage to cells than the other types of radiation
Alpha is the least penetrating type of radiation
This means it travels the shortest distance in air before being absorbed
Alpha particles have a range of around 3-7 cm in air
Alpha particles can be deflected slightly in strong electric and magnetic fields
Alpha particles have the highest charge, but also the greatest mass, so their high momentum means they deflect less than a beta particle (in a given field)
Properties of Beta Radiation
Beta is a moderately ionising type of radiation
This is due to it having a charge of ±1e
This means it can do some slight damage to cells (less than alpha but more than gamma)
Beta is a moderately penetrating type of radiation
Beta particles have a range of around 20 cm - 3 m in air, depending on their energy
Beta particles can be deflected through large angles by electric and magnetic fields
Beta particles typically travel at much greater speeds than alpha particles, but have much less mass, so they deflect significantly more than an alpha particle (in a given field)
Properties of Gamma Radiation
Gamma is the least ionising type of radiation
This is because it is an electromagnetic wave with no charge
This means it produces the least number of ion pairs per cm in air
It can still cause damage to cells, but not as much as alpha or beta radiation. This is why it is used for cancer radiotherapy
Gamma is the most penetrating type of radiation
This means it travels the furthest distance in air before being absorbed
Gamma radiation has an infinite range and follows an inverse square law
Gamma rays are not deflected in magnetic and electric fields as they are electrically neutral
However, they can transfer their energy to atomic electrons which can be deflected
Worked Example
Three successive radioactive decays are shown in the diagram below. Each decay results in a particle being emitted.

The first decay results in the emission of a beta-minus particle.
The second decay results in the emission of an alpha particle.
The third decay results in the emission of a beta-plus particle.
What is nuclide Z?
A.
B.
C.
D.
Answer: D
Step 1: Write the equation for the β− decay
A β− particle is an electron
The nucleon number stays the same
The proton number increases by 1
Step 2: Write the equation for the α decay
An α particle is a helium nucleus
The nucleon number reduces by 4
The proton number reduces by 2
Step 3: Write the equation for the β+ decay
A β+ particle is a positron
The nucleon number stays the same
The proton number reduces by 1
Step 4: Determine the final nucleon Z
The final nucleon, Z will be:

Unlock more, it's free!
Did this page help you?