The Photoelectric Effect (DP IB Physics): Revision Note
The Photoelectric Effect
The photoelectric effect is a phenomenon in which electrons are emitted from the surface of a metal upon the absorption of electromagnetic radiation
Electrons removed from the surface of a metal in this manner are known as photoelectrons
The photoelectric effect provides important evidence that light behaves as a particle i.e. it is quantised, or carried in discrete packets
This is shown by the fact each electron can absorb only a single photon
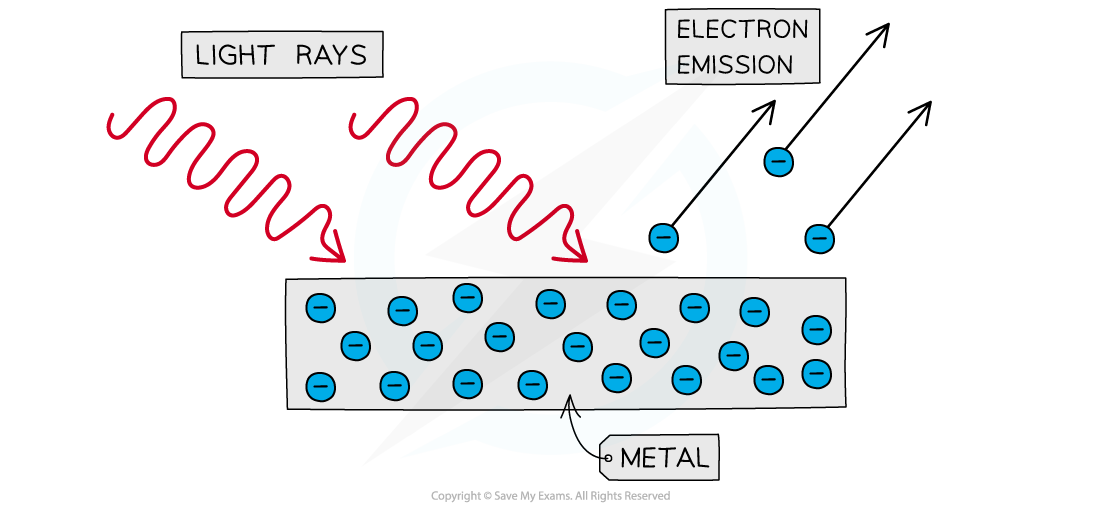
Photoelectrons are emitted from the surface of metal when light shines onto it
Threshold Frequency
Photoelectrons are emitted from the surface of a metal when light of sufficient energy shines on it
The frequency of the photons required for the photoelectric effect to occur is called the threshold frequency
The threshold frequency of a metal is defined as:
The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal
Threshold frequency and wavelength are properties of a material and vary from metal to metal
Threshold frequencies and wavelengths for different metals
Metal | Threshold Frequency | Threshold Wavelength |
|---|---|---|
sodium | 4.40 × 1014 | 682 |
potassium | 5.56 × 1014 | 540 |
zinc | 1.02 × 1015 | 294 |
iron | 1.04 × 1015 | 289 |
copper | 1.13 × 1015 | 266 |
gold | 1.23 × 1015 | 244 |
silver | 9.71 × 1015 | 30.9 |
Examiner Tips and Tricks
You are not required to memorise the threshold frequencies or wavelengths of different metals. These will be provided in the question if needed.
The Work Function
The work function Φ, or threshold energy, of a material is defined as:
The minimum energy required to release a photoelectron from the surface of a metal
Consider the electrons in a metal as trapped inside an ‘energy well’ where the energy between the surface and the top of the well is equal to the work function Φ
One electron absorbs one photon
Therefore, an electron can only escape from the surface of the metal if it absorbs a photon which has an energy equal to the work function Φ or higher
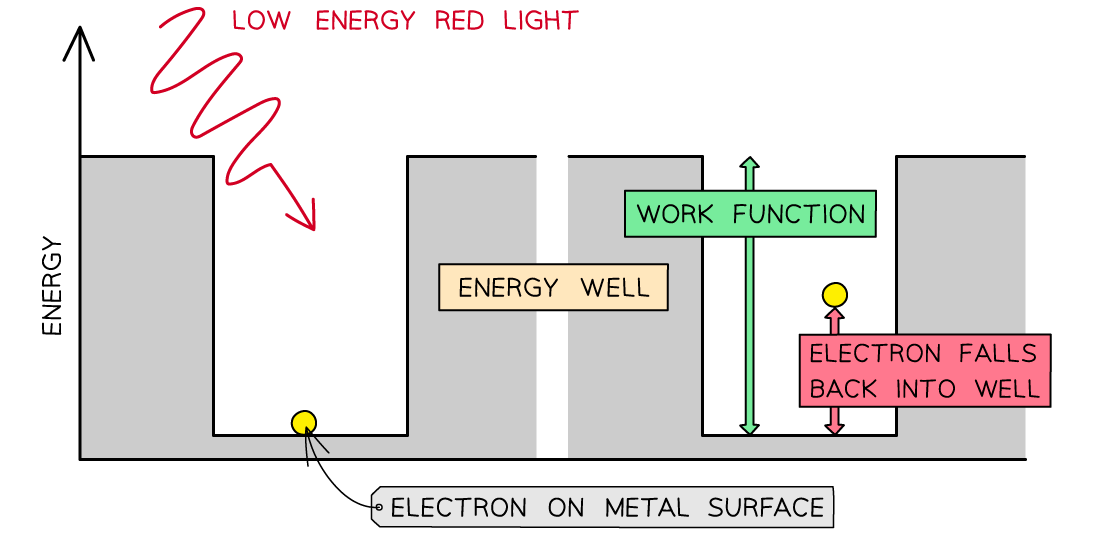
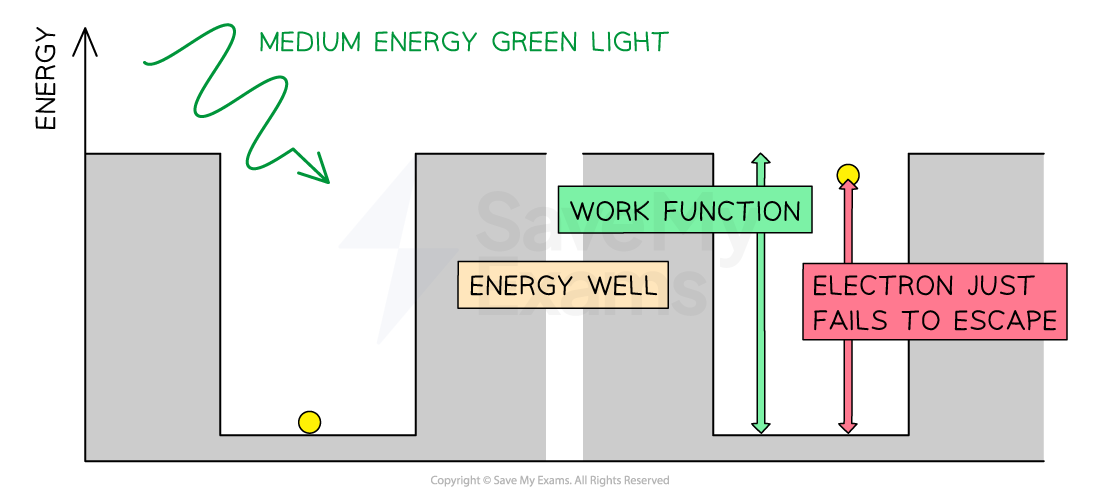
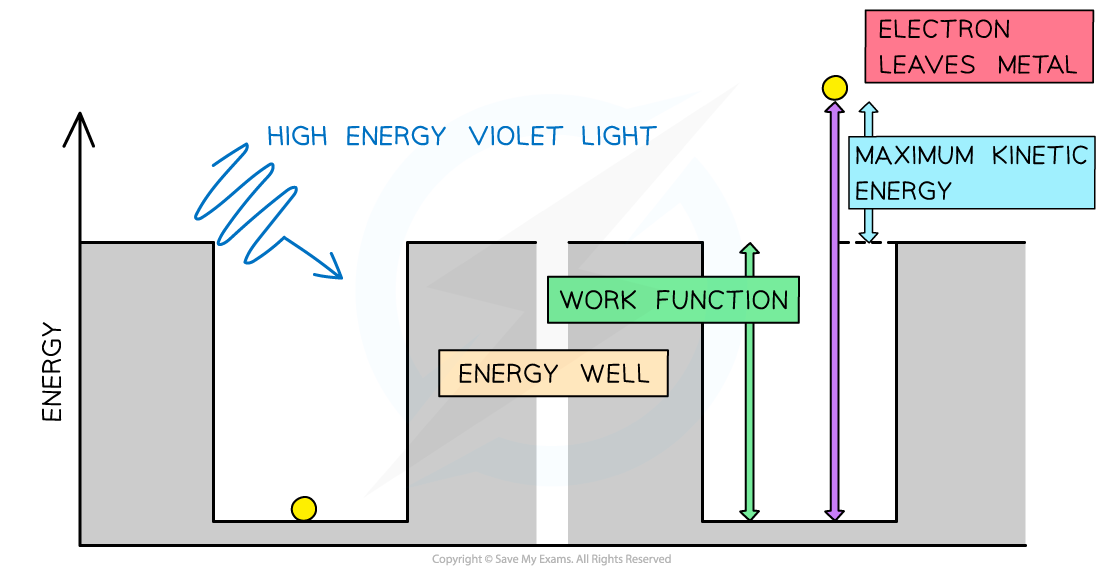
In the photoelectric effect, a single photon may cause a surface electron to be released if it has sufficient energy
Different metals have different threshold frequencies and hence different work functions
Using the well analogy:
A more tightly bound electron requires more energy to reach the top of the well
A less tightly bound electron requires less energy to reach the top of the well
Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region
This is because the attractive forces between the surface electrons and positive metal ions are relatively weak
Transition metals, such as zinc and iron, have threshold frequencies in the ultraviolet region
This is because the attractive forces between the surface electrons and positive metal ions are much stronger
Examiner Tips and Tricks
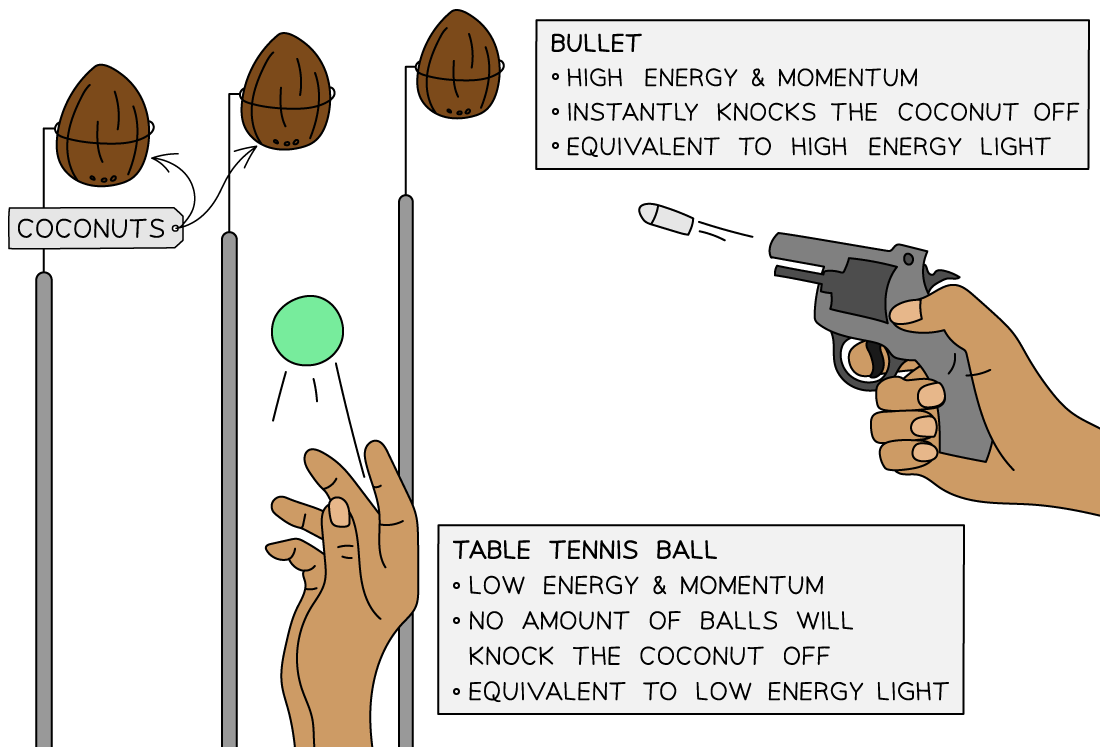
A useful analogy for threshold frequency is a fairground coconut shy:
One person is throwing table tennis balls at the coconuts, and another person has a pistol
No matter how many of the table tennis balls are thrown at the coconut it will still stay firmly in place – this represents the low frequency photons
However, a single shot from the pistol will knock off the coconut immediately – this represents the high frequency photons

Unlock more, it's free!
Did this page help you?