Mass Defect & Nuclear Binding Energy (DP IB Physics): Revision Note
Mass Defect & Nuclear Binding Energy
Experiments into nuclear structure have found that the total mass of a nucleus is less than the sum of the masses of its constituent nucleons
In other words, the combined mass of 6 separate protons and 6 separate neutrons is more than the mass of a carbon-12 nucleus
This difference in mass is known as the mass defect
Mass defect is defined as:
The difference between the measured mass of a nucleus and the sum total of the masses of its constituents
The mass defect Δm of a nucleus can be calculated using:
Where:
Z = proton number
A = nucleon number
mp = mass of a proton (kg)
mn = mass of a neutron (kg)
mtotal = measured mass of the nucleus (kg)
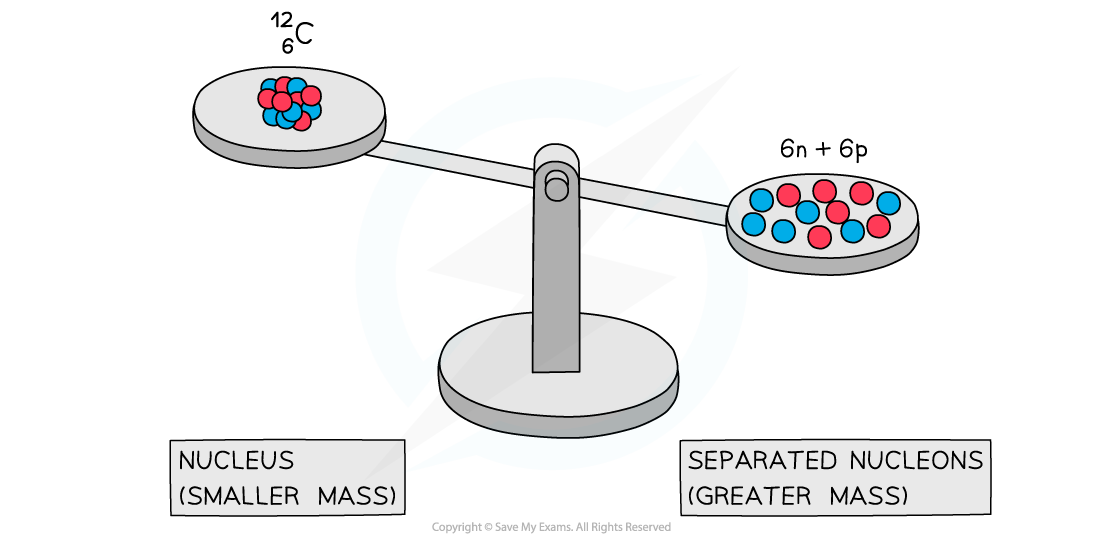
A system of separated nucleons has a greater mass than a system of bound nucleons
Due to mass-energy equivalence, a decrease in mass implies that energy must be released
Energy and mass are proportional, so the total energy of a nucleus is less than the sum of the energies of its constituent nucleons
Binding energy is defined as:
The energy required to break a nucleus into its constituent protons and neutrons
The formation of a nucleus from a system of isolated protons and neutrons releases energy
Worked Example
The binding energy per nucleon is 7.98 MeV for an atom of Oxygen-16 (16O).
Determine an approximate value for the energy required, in MeV, to completely separate the nucleons of this atom.
Answer:
Step 1: List the known quantities
Binding energy per nucleon, E = 7.98 MeV
Step 2: State the number of nucleons
The number of nucleons is 8 protons and 8 neutrons, therefore 16 nucleons in total
Step 3: Find the total binding energy
The binding energy for oxygen-16 is:
7.98 × 16 = 127.7 MeV
Step 4: State the final answer
The approximate total energy needed to completely separate this nucleus is 127.7 MeV
Examiner Tips and Tricks
The terms binding energy and mass defect can cause students confusion, so be careful when using them in your explanations.
Avoid describing the binding energy as the energy stored in the nucleus – this is not correct – it is energy that must be put into the nucleus to separate all the nucleons.
The same goes for the term mass defect, make sure to only use this when all the nucleons are separated and not to describe the decrease in mass which occurs during radioactive decay.
Mass-Energy Equivalence
Einstein showed in his Theory of Relativity that matter can be considered a form of energy, and hence, he proposed:
mass can be converted into energy
energy can be converted into mass
This is known as mass-energy equivalence, and can be summarised by the equation:
Where:
E = energy (J)
m = mass (kg)
c = the speed of light (m s-1)
Some examples of mass-energy equivalence are:
The fusion of hydrogen into helium in the centre of the sun
The fission of uranium in nuclear power plants
Nuclear weapons
High-energy particle collisions in particle accelerators
Atomic Mass Unit
The atomic mass unit is commonly used in nuclear physics to express the mass of subatomic particles
It is defined as:
Exactly one twelfth the mass of a neutral atom of carbon-12
Atomic mass unit u is roughly equal to the mass of one proton or neutron:
1 u = 1.661 × 10−27 kg
Using more precise values for well-known constants, a useful conversion factor can be determined
A particle with a mass of 1 u has an equivalent energy of
Converting to eV by using the precise value of the elementary charge gives
Therefore, the unified atomic mass unit can be used to quickly convert between nuclear mass and energy using:
1 u = 1.661 × 10−27 kg = 931.5 MeV c−2
Worked Example
Calculate the binding energy per nucleon, in MeV, for the radioactive isotope potassium-40 .
You may use the following data:
Nuclear mass of potassium-40 = 39.953 548 u
Mass of one neutron = 1.008 665 u
Mass of one proton = 1.007 276 u
Answer:
Step 1: Identify the number of protons and neutrons in potassium-40
Proton number, Z = 19
Neutron number, N = 40 – 19 = 21
Step 2: Calculate the mass defect, Δm
Proton mass, mp = 1.007 276 u
Neutron mass, mn = 1.008 665 u
Mass of potassium-40, mtotal = 39.953 548 u
Δm = Zmp + Nmn – mtotal
Δm = (19 × 1.007276) + (21 × 1.008665) – 39.953 548
Δm = 0.36666 u
Step 3: Convert mass units from u to kg
1 u = 1.661 × 10–27 kg
Δm = 0.36666 × (1.661 × 10–27) = 6.090 × 10–28 kg
Step 4: Write down the equation for mass-energy equivalence
E = Δmc2
Where c = 3.0 × 108 m s–1
Step 5: Calculate the binding energy, E
E = 6.090 × 10–28 × (3.0 × 108)2 = 5.5 × 10–11 J
Step 6: Determine the binding energy per nucleon and convert J to MeV
Take the binding energy and divide it by the number of nucleons
1 MeV = 1.6 × 10–13 J
Binding energy per nucleon = J
Binding energy per nucleon = = 8.594 MeV

Unlock more, it's free!
Was this revision note helpful?
