Fusion Reactions in Stars (DP IB Physics) : Revision Note
Fusion Reactions in Stars
In the centre of a stable star, hydrogen atoms undergo nuclear fusion to form helium
The equation for this reaction is:

Deuterium and tritium are both isotopes of hydrogen. They can be formed through other fusion reactions in the star
Fusion is defined as:
The joining of two small nuclei to produce a larger nucleus
Low-mass nuclei (such as hydrogen and helium) can undergo fusion and release energy
A huge amount of energy is released in the reaction
This provides a radiation pressure that prevents the star from collapsing under its gravity
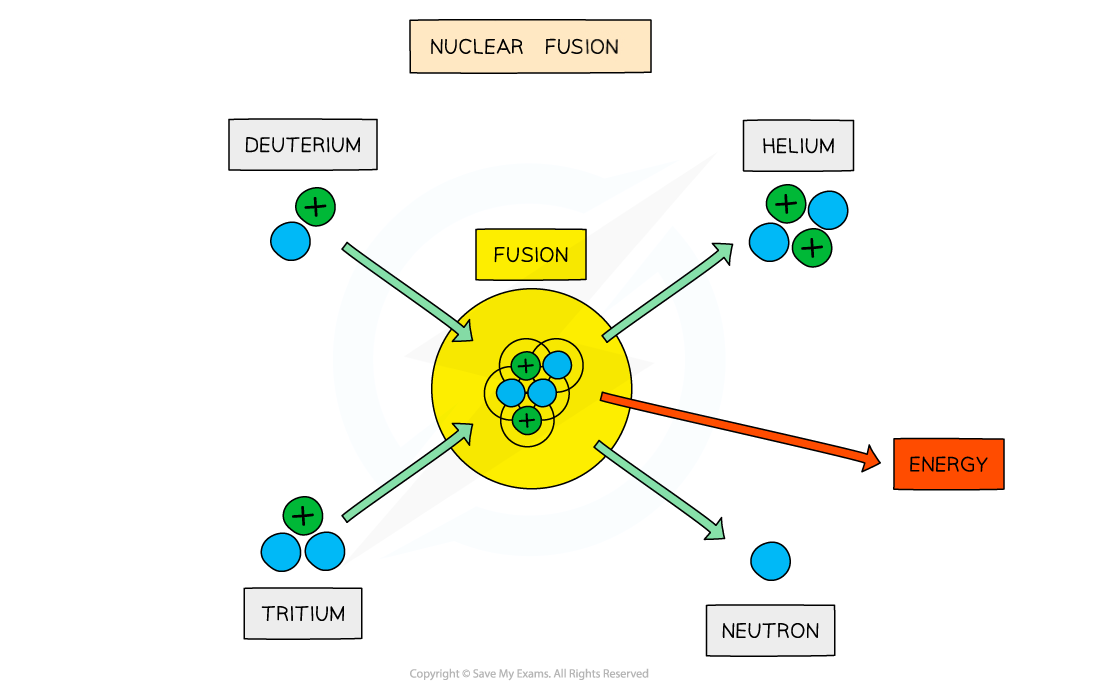
The fusion of deuterium and tritium to form helium with the release of energy
When two protons fuse, a deuterium nucleus is produced
In the centre of stars, the deuterium combines with a tritium nucleus to form a helium nucleus
The total mass of the helium nucleus is less than the total mass of the individual nucleons
Hence, the reaction releases energy, which provides fuel for the star to continue burning
Fusion & The Strong Nuclear Force
For two nuclei to fuse, both nuclei must have high kinetic energy
This is because
Nuclei must overcome the repulsive coulomb forces between protons
The strong nuclear force, which binds nucleons together, has a very short range
Therefore, nuclei must get very close together for the strong nuclear force to take effect
This means an extremely hot and dense environment is required to achieve fusion
Examiner Tips and Tricks
In the fusion process, the mass of the new heavier nucleus is less than the mass of the constituent parts of the nuclei fused together, as some mass is converted into energy.
Not all of this energy is used as binding energy for the new larger nucleus, so energy will be released from this reaction. The binding energy per nucleon afterwards is higher than at the start.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
