Energy Released in Fission Reactions (DP IB Physics) : Revision Note
Energy Released in Fission Reactions
When a large (parent) nucleus, such as uranium-235, undergoes a fission reaction, the daughter nuclei produced as a result will have a higher binding energy per nucleon than the parent nucleus
As a result of the mass defect between the parent nucleus and the daughter nuclei, energy is released
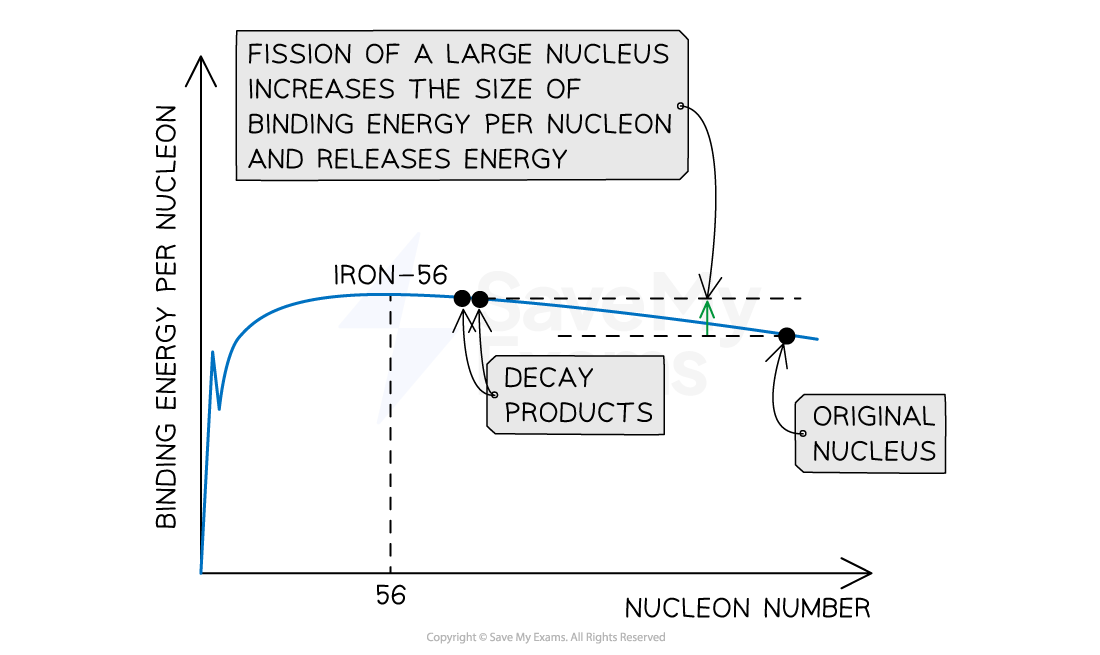
Energy can be extracted from fission reactions due to the mass defect between parent and daughter nuclei
Nuclear fission is well-regarded as having the fuel source with the highest energy density of any fuel that is currently available to us (until fusion reactions become feasible)
Examples of Common Fuels: Energy Density and Specific Energy Table
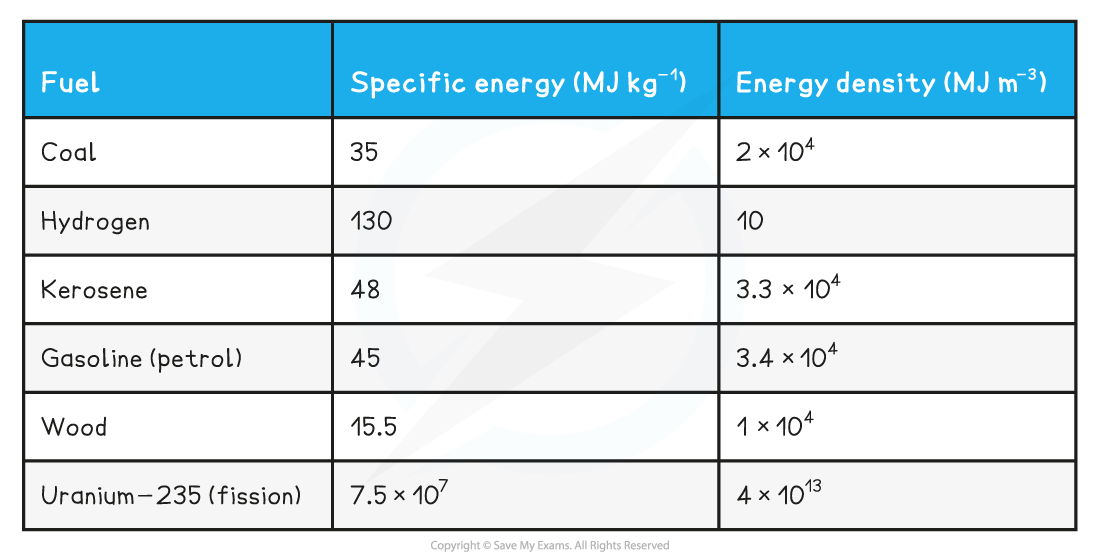
Calculations involving energy released in fission reactions often require the use of equations found in an array of previous topics, such as
Worked Example
When a uranium-235 nucleus absorbs a slow-moving neutron and undergoes a fission reaction, one possible pair of fission fragments is technetium-112 and indium-122.
The equation for this process, and the binding energy per nucleon for each isotope, are shown below.
nucleus | binding energy per nucleon / MeV |
7.59 | |
8.36 | |
8.51 |
(a) Calculate the energy released per fission of uranium-235, in MeV.
(b) Determine the mass of uranium-235 required per day to run a 500 MW power plant at 35% efficiency.
(c) The specific energy of coal is approximately 35 MJ kg−1.
For the same power plant, estimate the ratio
Answer:
(a) Energy released per fission of uranium-235
Step 1: Determine the binding energies of the nuclei before and after the reaction
Binding energy is equal to binding energy per nucleon × mass number
Binding energy before
= 235 × 7.59 = 1784 MeV
Binding energy after
= (112 × 8.36) + (122 × 8.51) = 1975 MeV
Step 2: Find the difference to obtain the energy released per fission reaction
Therefore, the energy released per fission = 1975 – 1784 = 191 MeV
(b) Mass of uranium-235 required per day
Step 1: List the known quantities
Avogadro's number, NA = 6.02 × 1023 mol−1
Molar mass of U-235, mr = 235 g mol−1
Power output, Pout = 500 MW = 500 × 106 J s−1
Efficiency, e = 35% = 0.35
Time, t = 1 day = 60 × 60 × 24 = 86 400 s
Step 2: Determine the number of atoms in 1 kg of U-235
There are NA (Avogadro’s number) atoms in 1 mol of U-235, which is equal to a mass of 235 g
number of atoms =
A mass of 1 kg (1000 g) of U-235 contains
= 2.562 × 1024 atoms kg−1
Step 3: Determine the specific energy of U-235
Specific energy of U-235 = total amount of energy released by 1 kg of U-235
Specific energy of U-235 = (number of atoms per kg) × (energy released per atom) = energy released per kg
Energy released per atom of U-235 = 191 MeV
Therefore, specific energy of U-235 = (2.562 × 1024) × 191 = 4.893 × 1026 MeV kg−1
To convert 1 MeV = 106 × (1.6 × 10−19) J
Specific energy of U-235 = (4.893 × 1026) × 106 × (1.6 × 10−19) = 7.83 × 1013 J kg−1
Step 4: Use the relationship between power, energy and efficiency to determine the mass
The input power required is:
efficiency & power:
input power:
input power:
Therefore, the mass of U-235 required in a day is:
mass of U-235 (per second) =
mass of U-235 (per day) = (1.82 × 10−5) × 86 400 = 1.58 kg
Therefore, 1.58 kg of uranium-235 is required per day to run a 500 MW power plant at 35% efficiency
(c) Ratio of the masses of coal and U-235
Since specific energy
Where the energy density of coal = 35 MJ kg−1
Over 2 million times (~3.5 × 106 kg) more coal is required than uranium-235 to achieve the same power output in a day (or second, or month or year)
Examiner Tips and Tricks
If you need to brush up on binding energy calculations, take a look at the Mass Defect & Nuclear Binding Energy revision notes.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
