Millikan's Oil Drop Experiment (DP IB Physics): Revision Note
Millikan's Oil Drop Experiment
This experiment was conducted by Millikan and Fletcher in 1909
It determined the value of the fundamental elementary charge
Method for Millikan's Oil Drop Experiment
A fine mist of oil drops is sprayed into a chamber
Oil is used instead of water because it does not evaporate quickly
This means the mass of the drops will remain constant
As the drops pass out of the spray nozzle they are charged by friction (alternatively, they can also be ionised by X-rays)
Some drops lose electrons and become positively charged
Some drops gain electrons and become negatively charged
The drops pass into a region between two metal plates and are viewed using a microscope
Equipment Set Up for Millikan's Oil Drop Experiment
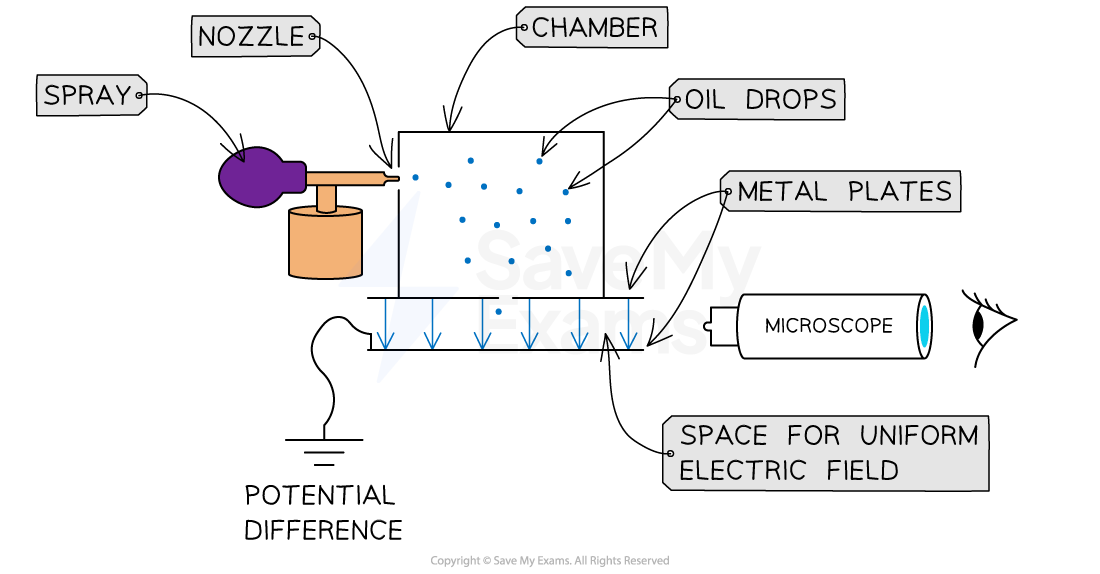
In Millikan's Oil Drop Experiment oil is sprayed into a chamber before passing between metal plates where the electric and gravitational forces are compared
Electric vs Gravitational Force
No Electric Field
The oil drops fall under gravity between the metal plates
They reach a terminal velocity when the air resistance and gravitational force acting on the drop are equal
With Electric Field
A potential difference is applied between the metal plates which creates an electric field
The charged oil drops begin to rise when the electric field is strong enough
This means the upward electrical force is greater than the gravitational force
The equation for electric force is:
Where:
E = electric field strength (N C-1)
F = electrostatic force on the charge (N)
q = charge (C)
The distance the drops rise depends upon their mass
With the correct potential difference applied, the electric and gravitational forces can become equal and opposite
The equation for gravitational force, which comes from Newton's second law, is:
Where:
W = weight of drop (N)
m = mass of drop (kg)
g = gravitational field strength (N kg−1)
By equating the electric and gravitational forces of the drops, the value of fundamental charge was determined to be 1.60 × 10−19 C
The magnitude of the charge on any object is found to be a multiple of 1.60 × 10−19 C
Therefore, Millikan's experiment provides evidence for the quantisation of charge

Unlock more, it's free!
Was this revision note helpful?
