Thermodynamic Processes (DP IB Physics): Revision Note
Thermodynamic Processes
The four main thermodynamic processes are
isovolumetric
isobaric
isothermal
adiabatic
Note: In all processes, the gas is assumed to be ideal
Constant pressure (isobaric)
An isobaric process is defined as:
A process in which no change in pressure occurs
This occurs when gases are allowed to expand or contract freely during a change in temperature
When there is a change in volume ΔV at a constant pressure p, work done W is equal to
From the first law of thermodynamics:
The ± sign reflects whether work has been done on or by the gas as a result of the change in volume
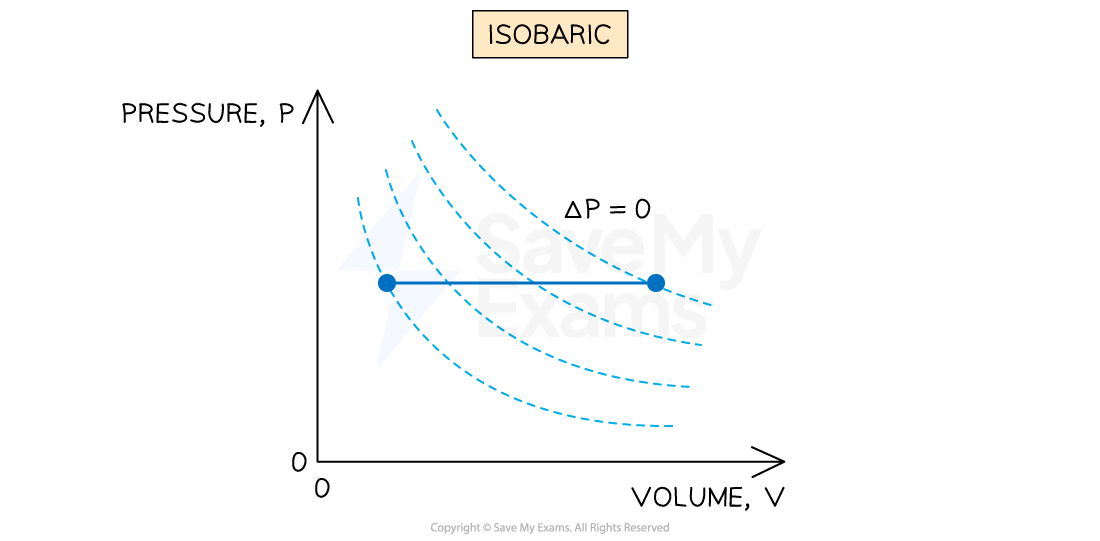
Representing an isobaric process on a p-V diagram
Constant volume (isovolumetric)
An isovolumetric process is defined as:
A process where no change in volume occurs and the system does no work
If there is no change in volume, then there is no work done on or by the gas, so
Therefore, from the first law of thermodynamics:
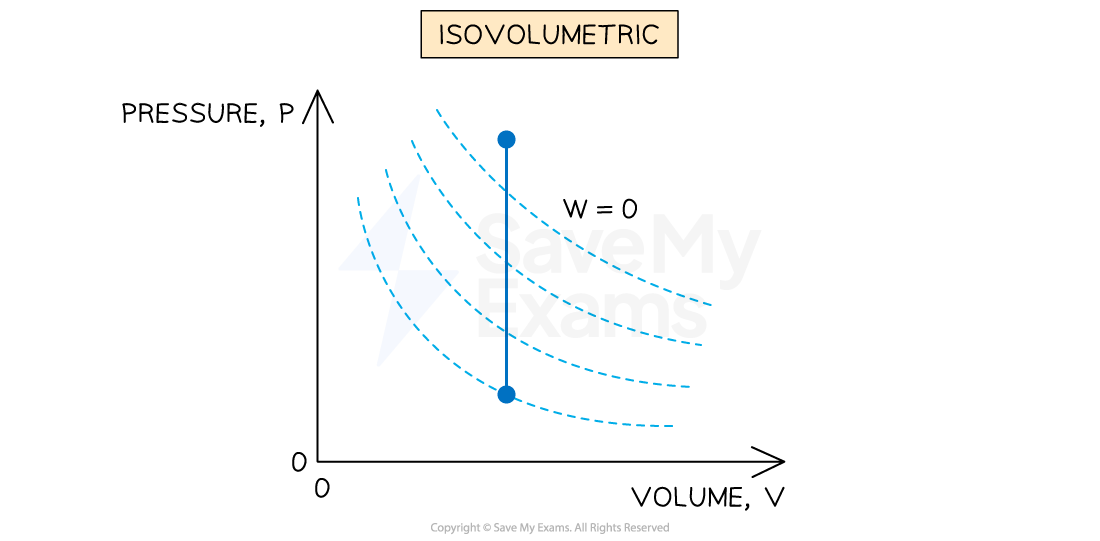
Representing an isovolumetric process on a p-V diagram
Constant temperature (isothermal)
An isothermal process is defined as:
A process in which no change in temperature occurs
If the temperature does not change, then the internal energy of the gas will not change, so
Therefore, from the first law of thermodynamics:
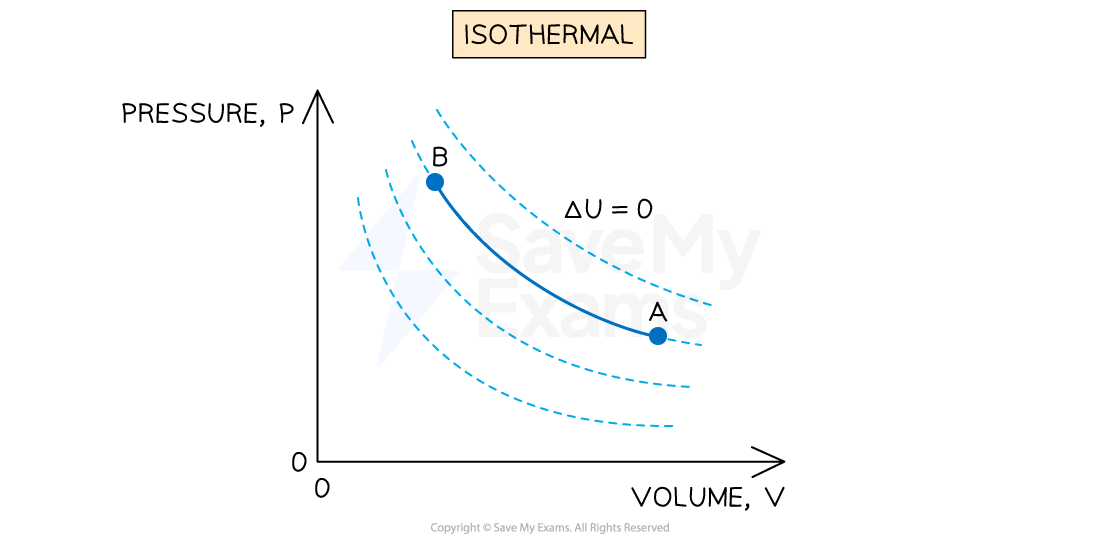
Representing an isothermal process on a p-V diagram
Constant thermal energy (adiabatic)
An adiabatic process is defined as:
A process where no heat is transferred into or out of the system
If there is no heat entering or leaving the system then
Therefore, from the first law of thermodynamics:
This means that all the work done is at the expense of the system's internal energy
Hence, an adiabatic process will usually be accompanied by a change in temperature
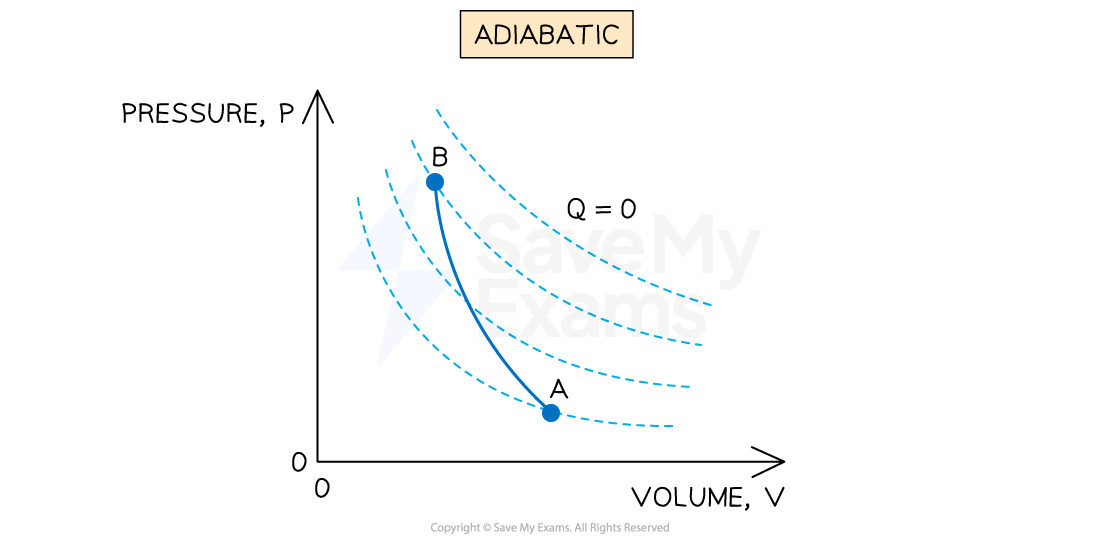
Representing an adiabatic process on a p-V diagram
Entropy in thermodynamic processes
At a constant temperature T, the change in entropy is related to heat by
When heat is gained by a system
, entropy increases
When heat is lost from a system
, entropy decreases
For a reversible process
that returns the system to its original state
Process | Heat gained or lost, ΔQ | Change in entropy, ΔS | |
|---|---|---|---|
Isothermal | Expansion | Heat gained = work done by gas | Increases |
Compression | Heat lost = work done on gas | Decreases | |
Isobaric | Expansion | Heat gained = increase in internal energy + work done by gas | Increases |
Compression | Heat lost = decrease in internal energy + work done on gas | Decreases | |
Isovolumetric | Pressure rise | Heat gained due to temperature rise | Increases |
Pressure drop | Heat lost due to temperature drop | Decreases | |
Adiabatic | Expansion | Pressure & temperature decrease with no heat gained or lost | No change |
Compression | Pressure & temperature increase with no heat gained or lost | No change | |
Worked Example
A quantity of energy Q is supplied to three ideal gases, X, Y and Z.
Gas X absorbs Q isothermally, gas Y isovolumetrically and gas Z isobarically.
Complete the table by inserting the words ‘positive’, ‘zero’ or ‘negative’ for the work done W, the change in internal energy ΔU and the temperature change ΔT for each gas.
| |||
|---|---|---|---|
X |
|
|
|
Y |
|
|
|
Z |
|
|
|
Answer:
X: Isothermal = constant temperature, no change in internal energy
Temperature:
Internal energy:
, so,
Work done:
Y: Isovolumetric = constant volume, no work done
Work done:
, so,
Internal energy:
Temperature:
, so,
Z: Isobaric = constant pressure
Work done:
, so
, so
Internal energy:
, so
Temperature:
, so
| |||
|---|---|---|---|
X | positive | 0 | 0 |
Y | 0 | positive | positive |
Z | positive | positive | positive |
Worked Example
A heat engine operates on the cycle shown in the pressure-volume diagram. One step in the cycle consists of an isothermal expansion of an ideal gas from state A of volume V to state B of volume 2V.
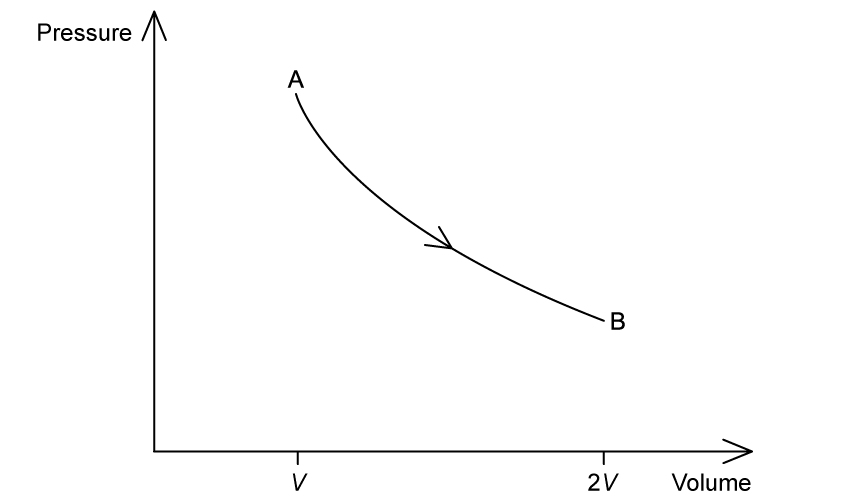
(a) On the graph, complete the cycle ABCA by drawing curves to show
an isovolumetric change from state B to state C
an adiabatic compression from state C to state A
(b) State and explain at which point in the cycle ABCA the entropy of the gas is the largest.
Answer:
(a)
Isovolumetric = constant volume, no work done
Next step is a compression (where pressure increases), so this step should involve a pressure drop
Hence, B to C: line drawn vertically down
Adiabatic = no heat supplied or removed, compression = work is done on the gas, volume decreases
Hence, C to A: line curves up to meet A
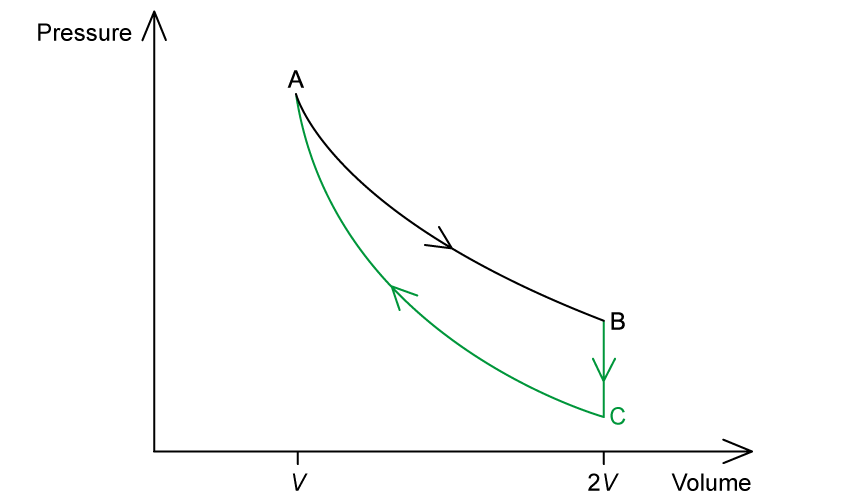
(b)
Entropy and heat (at a constant T) are related by
From state A to state B:
In an isothermal expansion, entropy increases
Because T = constant but the volume increases so work is done by gas, ΔQ > 0 so ΔS > 0
From state B to state C:
In an isovolumetric change where pressure decreases, entropy decreases
Because temperature decreases, so energy has been removed, ΔQ < 0 so ΔS < 0
From state C to state A:
In an adiabatic compression, entropy is constant
Because it is an adiabatic process, ΔQ = 0 so ΔS = 0
Therefore, entropy is greatest at B
Adiabatic Processes
Adiabatic processes in monatomic ideal gases can be modelled by the equation
Where:
p = pressure of the gas (Pa)
V = volume occupied by the gas (m3)
This equation can be used for calculating changes in pressure, volume and temperature for monatomic ideal gases
Where:
= initial pressure (Pa)
= final pressure (Pa)
= initial volume (m3)
= final volume (m3)
Worked Example
An ideal monatomic gas expands adiabatically from a state with pressure 7.5 × 105 Pa and volume 1.8 × 10−3 m3 to a state of volume 4.2 × 10−3 m3.
Calculate the new pressure of the gas.
Answer:
For an ideal monatomic gas undergoing an adiabatic change:
Where:
Initial pressure,
= 7.5 × 105 Pa
Final pressure =
Initial volume,
= 1.8 × 10−3 m3
Final volume,
= 4.2 × 10−3 m3
New pressure: = 1.8 × 105 Pa
Worked Example
An ideal monatomic gas is compressed adiabatically from a state with volume 3.1 × 10−3 m3 and temperature 590 K to a state of volume 2.1 × 10−3 m3.
Calculate the new temperature of the gas.
Answer:
For an ideal monatomic gas undergoing an adiabatic change:
From the ideal gas law:
Where:
Initial temperature,
= 590 K
Final temperature =
Initial volume,
= 3.1 × 10−3 m3
Final volume,
= 2.1 × 10−3 m3
New temperature: = 765 K

Unlock more, it's free!
Did this page help you?