First Law of Thermodynamics (DP IB Physics): Revision Note
The First Law of Thermodynamics
The first law of thermodynamics is based on the principle of conservation of energy
When energy is put into a gas by heating it or doing work on it, its internal energy must increase:
energy supplied by heating = change in internal energy + work done on the system
The first law of thermodynamics is therefore defined as:
Where:
Q = energy supplied to the system by heating (J)
ΔU = change in internal energy (J)
W = work done by the system (J)
The first law of thermodynamics applies to all situations, not just to gases
There is an important sign convention used for this equation
A positive value for internal energy (+ΔU) means:
The internal energy ΔU increases
Heat Q is added to the system (+Q)
Work W is done on the system (–W)
A negative value for internal energy (−ΔU) means:
The internal energy ΔU decreases
Heat Q is taken away from the system (–Q)
Work W is done by the system (+W)
Graphs of Constant Pressure & Volume
Graphs of pressure p against volume V can provide information about the work done and internal energy of the gas
The work done is represented by the area under the line
A constant pressure process is represented as a horizontal line
If the volume is increasing (expansion), work is done by the gas (on the surroundings) and internal energy decreases (ΔU = q − W)
If the arrow is reversed and the volume is decreasing (compression), work is done on the gas and internal energy increases (ΔU = q + W)
The volume of the gas is made smaller, so more collisions between the molecules of the gas and the walls of the container occur. This creates a higher pressure.
A constant volume process is represented as a vertical line
In a process with constant volume, the area under the curve is zero
Therefore, no work is done when the volume stays the same
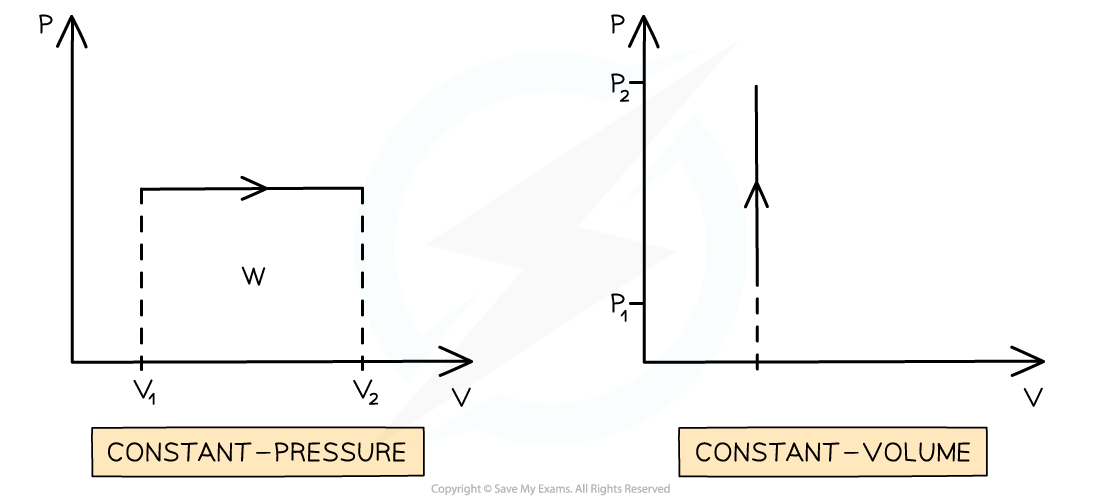
Work is only done when the volume of a gas changes
Worked Example
The volume occupied by 1.00 mol of a liquid at 50°C is 2.4 × 10−5 m3. When the liquid is vaporised at an atmospheric pressure of 1.03 × 105 Pa, the vapour occupies a volume of 5.9 × 10−2 m3.
The latent heat to vaporise 1.00 mol of this liquid at 50°C at atmospheric pressure is 3.48 × 104 J.
For this change of state, determine the increase in internal energy ΔU of the system.
Answer:
Step 1: List the known quantities
Thermal energy, Q = 3.48 × 104 J
Atmospheric pressure, p = 1.03 × 105 Pa
Initial volume = 2.4 × 10−5 m3
Final volume = 5.9 × 10−2 m3
Step 2: Calculate the work done W
The work done by a gas at constant pressure is
Where the change in volume is:
ΔV = final volume − initial volume = (5.9 × 10−2) − (2.4 × 10−5) = 0.059 m3
Since the volume of the gas increases, the work done is positive
W = (1.03 × 105) × 0.059 = 6077 = 6.08 × 103 J
W = +6.08 × 103 J
Step 3: Substitute the values into the equation for the first law of thermodynamics
From the first law of thermodynamics:
ΔU = (3.48 × 104) − (6.08 × 103) = 28 720 J

Unlock more, it's free!
Did this page help you?