Calculating Changes in Entropy (DP IB Physics): Revision Note
Calculating Changes in Entropy
At a constant temperature T, the change in entropy on a macroscopic level can be calculated using the equation
Where:
ΔS = change in entropy (J K−1)
ΔQ = heat given to or removed from the system (J)
T = temperature of the system (K)
When heat is given to a system (ΔQ = positive), entropy increases
When heat is removed from a system (ΔQ = negative), entropy decreases
For a reversible process that returns the system to its original state, entropy is constant
Entropy & Microstates
The entropy of a system, on a microscopic level, can be calculated using the equation
Where:
S = entropy of a system of microscopic particles (J K−1)
kB = the Boltzmann constant
Ω = the number of possible microstates of the system
Similarly, the change in entropy when the number of microstates increases from
to
is given by
A microstate describes one state or possible arrangement of the particles in the system
A state can be defined by any microscopic or macroscopic property that is known about the system e.g. positions or velocities of molecules, energy, volume etc.
An example that helps illustrate this is a two-compartment container which holds N distinguishable particles (i.e. each particle can be identified individually)
Initially, all N particles are sealed in one of two compartments
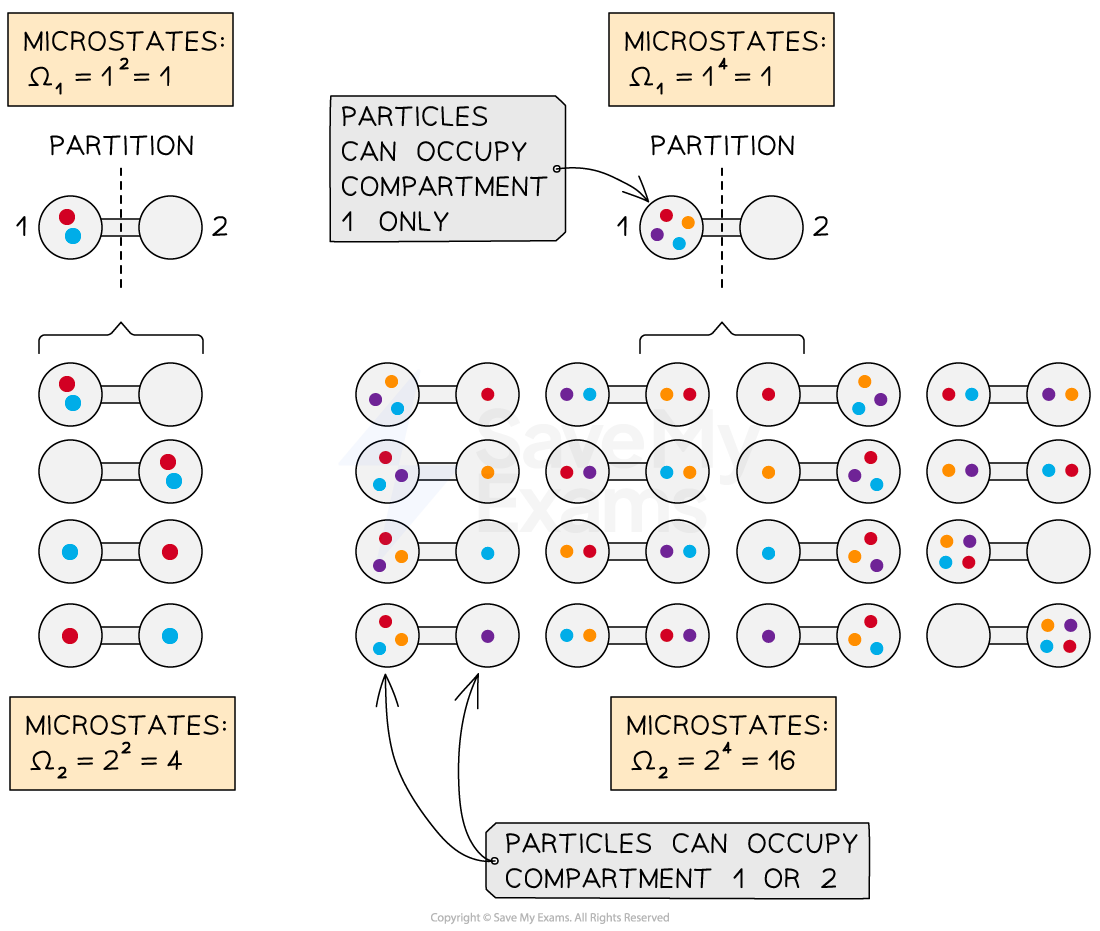
The number of possible microstates describes the number of different possible arrangements of particles in a system
When the particles are confined to one compartment, we know the location of all the particles
Therefore, the number of microstates (possible arrangements) in the initial volume is
It is always equal to 1, for example, when N = 2 or N = 4:
Once the partition is removed, the particles can spread out and occupy either one of the two compartments
The number of microstates (possible arrangements) in the final volume is
For example, when N = 2, the particles can be arranged 22 = 4 different ways
Or, when N = 4, the particles can be arranged 24 = 16 different ways
The change in the entropy is therefore:
It follows that the number of possible microstates can be equated to macroscopic properties of the gas, such as its volume increasing from V to 2V
As the gas expands, the space it can occupy doubles, hence it gains an amount of entropy equal to:
This gives the same result as above:
Examiner Tips and Tricks
Entropy is an incredibly important topic in physics and underpins many fundamental ideas from quantum mechanics to the determination of the Schwarzchild radius of a black hole, so don't worry if you feel a bit lost at first as it is quite a challenging concept to get your head around initially!
You might find it useful to think of microstates as a way of quantifying the certainty of information we have about the system
For example:
A solid has lower entropy than a gas because we can be more certain about the location of the atoms in the solid
A gas at a higher temperature (or pressure or volume) has a higher entropy than a similar gas at a lower temperature (or pressure or volume) because we become less certain about the location of the atoms by further increasing the possible locations they could occupy

Unlock more, it's free!
Did this page help you?