Temperature & Kinetic Energy (DP IB Physics): Revision Note
Temperature & Kinetic Energy
The molecules in a gas usually have a range of speeds
For an ideal gas, the average kinetic energy
of the molecules can be calculated using the equation
Where:
= average kinetic energy of the molecules in joules (J)
= 1.38 × 10–23 J K–1 (Boltzmann's constant)
= absolute temperature of the gas in kelvin (K)
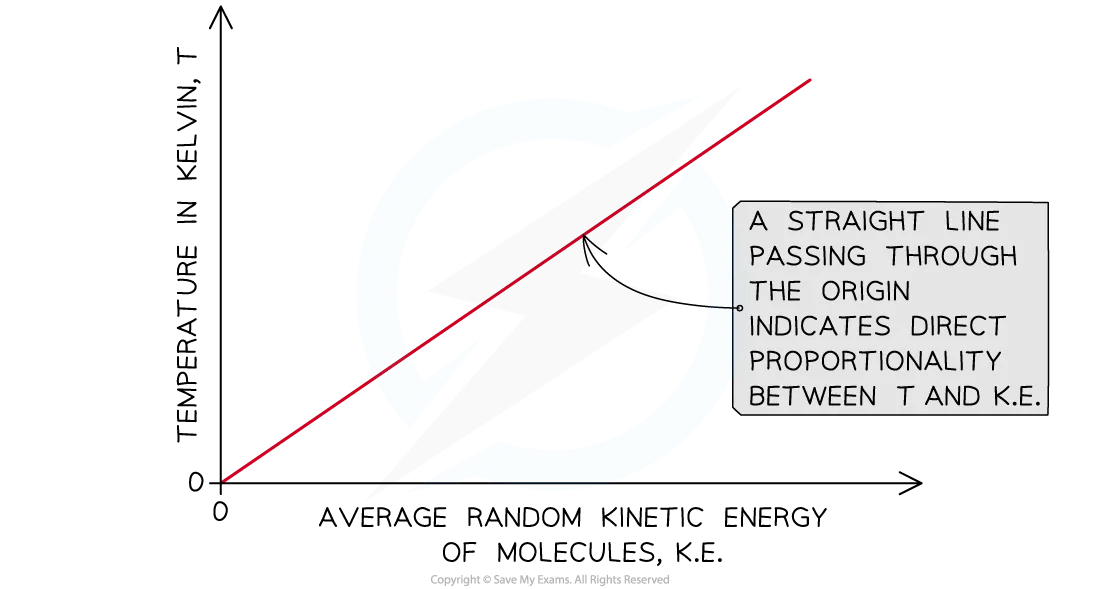
This tells us that the absolute temperature of an ideal gas is directly proportional to the average kinetic energy of the molecules within it
Relationship between absolute temperature and average random kinetic energy of molecules
Worked Example
The surface temperature of the Sun is 5800 K and contains mainly hydrogen atoms.
Calculate the average speed of the hydrogen atoms, in km s−1, near the surface of the Sun.
Answer:
Step 1: List the known quantities
Temperature, T = 5800 K
Mass of a hydrogen atom = mass of a proton, mp = 1.673 × 10−27 kg
Boltzmann constant, kB = 1.38 × 10−23 J K−1
Step 2: Equate the equations relating kinetic energy with temperature and speed
Average kinetic energy of the molecules:
Kinetic energy:
Step 3: Rearrange for average speed and calculate
Average speed: v = 11 980 m s−1 = 12 km s−1

Unlock more, it's free!
Did this page help you?