Specific Latent Heat (DP IB Physics): Revision Note
Specific Latent Heat
During a phase change (i.e. a change of state) thermal energy is transferred to a substance or removed from it
During a phase change, the temperature of the substance does not change
In this case, the thermal energy is calculated as follows:
Where:
Q = heat energy transferred (J)
m = mass of the substance in kilograms (kg)
L = specific latent heat of the substance (J kg–1)
The specific latent heat of a substance is defined as:
The amount of energy required to change the state of 1 kg of a substance without changing its temperature
This definition can be explained when the above equation is rearranged for L:
This means that the higher the specific latent heat of a substance, the greater the energy needed to change its state
Note that the specific latent heat is measured in J kg–1
The amount of energy required to melt (or solidify) a substance is not the same as the amount of energy required to evaporate (or condense) the same substance
Hence, there are two types of specific heat:
Specific latent heat of fusion, Lf
Specific latent heat of vaporisation, Lv
Specific Latent Heat of Fusion
Specific latent heat of fusion is defined as:
The energy released when 1 kg of liquid freezes to become solid at constant temperature
OR
The energy absorbed when 1 kg of solid melts to become liquid at constant temperatureThis is because fusion applies to the following phase changes:
Solid to liquid
Liquid to solid
Specific Latent Heat of Vaporisation
Specific latent heat of vaporisation is defined as:
The energy released when 1 kg of gas condenses to become liquid at constant temperature
OR
The energy absorbed when 1 kg of liquid evaporates to become gas at constant temperatureThis is because vaporisation applies to the following phase changes:
Liquid to gas
Gas to liquid
What is the difference between the latent heat of vaporisation and fusion?
For a given substance, the value of the specific latent heat of vaporisation is always higher than the value of the specific latent heat of fusion
In other words, Lv > Lf
This means more energy is required to evaporate (or condense) a substance than is needed to melt it (or solidify it)
During melting (fusion):
The intermolecular forces of attraction only need to be partially overcome to turn from a solid to a liquid
During evaporation (vaporisation):
The intermolecular forces of attraction need to be completely overcome to turn from liquid to gas
This requires a lot more energy
Substance | Specific Latent Heat of Fusion (J kg−1) | Specific Latent Heat of Vaporisation (J kg−1) |
|---|---|---|
Water | 4.0 × 105 | 1.1 × 107 |
Aluminium | 3.3 × 105 | 2.3 × 106 |
Copper | 2.1 × 105 | 4.7 × 106 |
Gold | 6.3 × 104 | 1.7 × 106 |
Worked Example
Determine the energy needed to melt 200 g of ice at 0°C.
The specific latent heat of fusion of water is 3.3 × 105 J kg–1
The specific latent heat of vaporisation of water is 2.3 × 106 J kg–1
Answer:
Step 1: Determine whether to use latent heat of fusion or vaporisation
We need to use the specific latent heat of fusion because the phase change occurring is from solid to liquid
Step 2: List the known quantities
Mass of the ice, m = 200 g = 0.2 kg
Specific latent heat of fusion of water, Lf = 3.3 × 105 J kg–1
Step 3: Write down the equation for the latent heat of fusion
Step 4: Substitute numbers into the equation
Q = 0.2 × (3.3 × 105)
Q = 66 000 = 66 kJ
Worked Example
Energy is supplied to a heater at a rate of 2500 W.
Determine the time taken, in minutes, to boil 500 ml of water at 100°C. Ignore energy losses.
The specific latent heat of fusion of water is 3.3 × 105 J kg–1
The specific latent heat of vaporisation of water is 2.3 × 106 J kg–1
Answer:
Step 1: Determine whether to use latent heat of fusion or vaporisation
We need to use the specific latent heat of vaporisation because the phase change occurring is from liquid to gas
Step 2: Write down the known quantities
Power, P = 2500 W
Mass, m = 500 ml = 0.5 kg (since1 litre = 1 kg)
Specific latent heat of vaporisation of water, Lv = 2.3 × 106 J kg–1
Step 3: Recall the equations for power and latent heat of
Power:
Thermal energy:
Step 4: Equate the two expressions for energy
Step 5: Rearrange for the time t and substitute in the values
t = 460 s = 7.7 min
Heating & Cooling Curves
A heating or cooling curve shows how the temperature of a substance changes with time
The two main features of these curves are
The flat sections
The non-flat sections
The flat sections show...
No change in temperature over time
The substance is undergoing a phase change
The thermal energy supplied to or removed from the substance only affects the potential energy of the particles
The non-flat sections show...
Changes in temperature over time
The substance is heating up or cooling down
That the thermal energy supplied to or removed from the substance changes the average kinetic energy of the particles, hence resulting in an overall change in the temperature of the substance
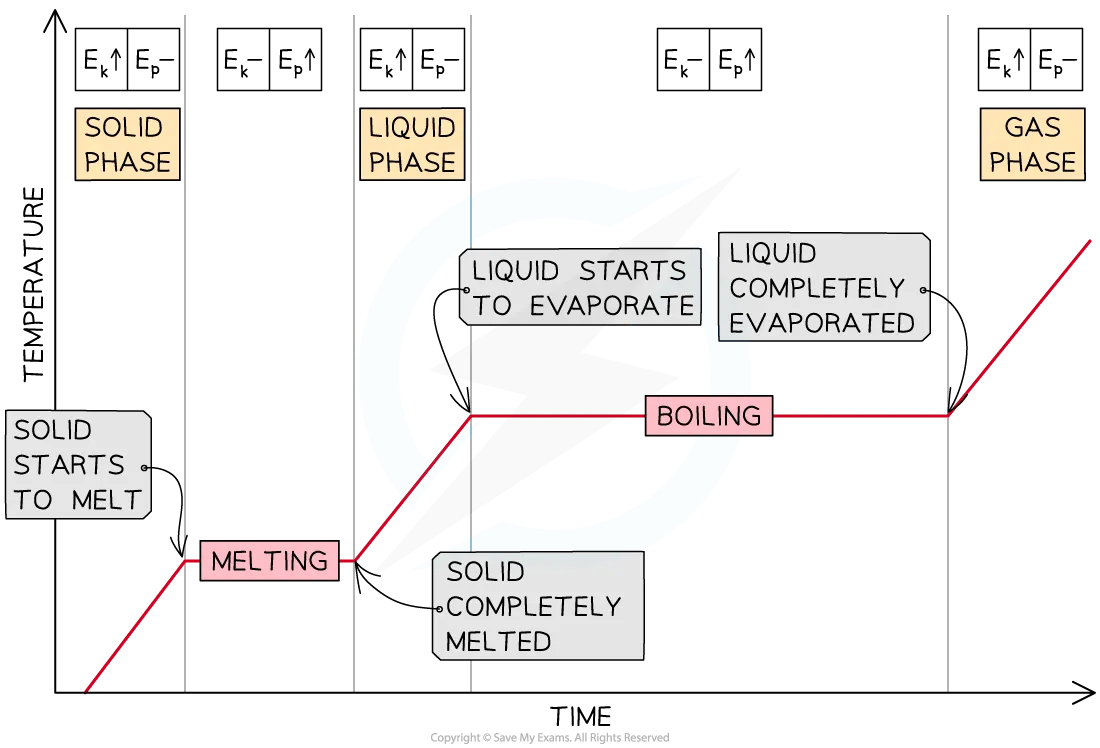
Heating Curves
When energy is supplied to a solid, its temperature will increase until it reaches its melting point
Once at the melting point, the temperature remains constant until the substance has melted completely
If energy continues to be supplied, the temperature of the liquid will increase until it reaches its boiling point
Once at the boiling point, the temperature remains constant until the substance has vaporised completely
Heating Curve of a Substance
Cooling Curves
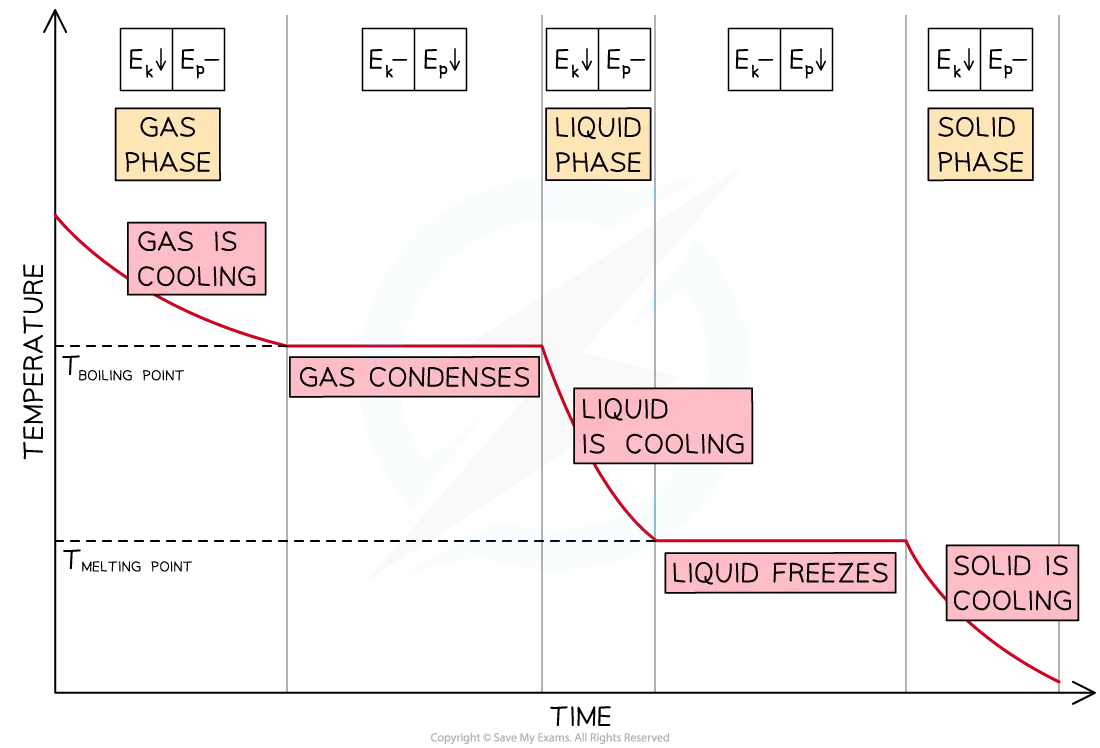
When energy is removed from a gas, its temperature will decrease until it reaches its boiling point
Once at the boiling point, the temperature remains constant until the substance has condensed completely
If energy continues to be removed, the temperature of the liquid will decrease until it reaches its freezing point
Once at the freezing point, the temperature remains constant until the substance has frozen completely
Cooling Curve of a Substance
Heating or cooling curves can also display how the temperature of a substance changes with energy
In the following worked example, energy (in J) is plotted on the x-axis instead of time
Worked Example
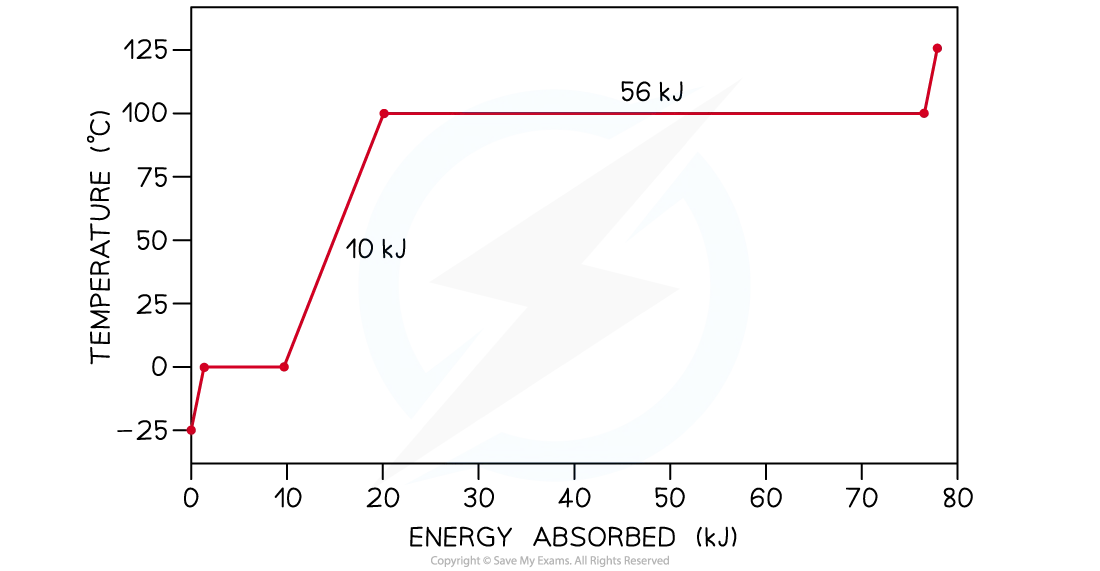
The graph below is the heating curve for a 24 g cube of ice being heated at a constant rate.
Using the graph, calculate
(a) The specific heat capacity of water in its liquid phase
(b) The specific latent heat of vaporisation of water
Answer:
Write down the known quantities:
Mass of the ice, m = 24 g = 0.024 kg
Melting point of water = 0°C
Boiling point of water = 100°C
Change in temperature of liquid water, ΔT = 100°C
(a)
Step 1: Identify the liquid phase on the graph and read off the amount of energy absorbed
The non-flat region between 0°C and 100°C represents water in its liquid phase being heated up
Energy absorbed by liquid water = 10 kJ = 10 000 J
Step 2: Write down the equation for specific heat capacity and rearrange for c
Step 3: Substitute in the values to calculate the specific heat capacity of water c
c = 4167 = 4200 J kg–1 °C–1
(b)
Step 1: Identify the phase change of vaporisation on the graph and read off the amount of energy absorbed
The second flat section at 100°C indicates the phase change from water into steam (vaporisation)
Energy absorbed during vaporisation = 56 kJ = 56 000 J
Step 2: Write down the equation for specific latent heat of vaporisation and rearrange for Lv
Step 3: Substitute in the values to calculate specific latent heat of vaporisation of water Lv
Lv = 2.3 × 106 J kg–1

Unlock more, it's free!
Did this page help you?