Rutherford's Gold Foil Experiment
- Evidence for the structure of the atom was discovered by Ernest Rutherford at the beginning of the 20th century from the study of alpha particle scattering
- The experiment consisted of beams of high-energy alpha particles fired at thin gold foil and a detector on the other side to determine
- The different angles of deflection of the alpha particles
- The number of alpha particles that were deflected at each angle
Apparatus for the Rutherford Scattering Experiment
- The setup for the scattering experiment consisted of:
- A source of alpha particles in a lead container
- A thin sheet of gold foil
- A movable detector
- An evacuated chamber

Experimental set up for α-particle scattering
Purpose of the lead container
- Alpha particles are emitted in all directions, so the source was placed in a lead container
- This was to produce a collimated beam of alpha particles
- This is because alpha particles are absorbed by lead, so a long narrow hole at the front allowed a concentrated beam of alpha particles to escape and be directed as needed
Purpose of the thin sheet of gold foil
- The target material needed to be extremely thin, about 10−6 m thick
- This is because a thicker foil would stop the alpha particles completely
- Gold was chosen due to its malleability, meaning it was easy to hammer into thin sheets
Purpose of the evacuated chamber
- Alpha particles are highly ionising, meaning they only travel about 5 cm before interacting with molecules of air
- So, the apparatus was placed in an evacuated chamber
- This was to ensure that the alpha particles did not collide with any particles on their way to the foil target
Findings from the Rutherford Scattering Experiment
- An alpha (α) particle is the nucleus of a helium atom, so it has a positive charge

When α-particles are fired at thin gold foil, most of them go straight through but a small number bounce straight back
- The observations from Rutherford's experiment were:
A. The majority of α-particles passed straight through the foil undeflected
-
- This suggests the atom is mostly empty space
B. Some α-particles deflected through small angles of <10°
-
- This suggests there is a positive nucleus at the centre (since two positive charges would repel)
C. Only a small number of α-particles deflected straight back at angles of >90°
-
- This suggests the nucleus is extremely small and is where most of the mass and charge of the atom are concentrated
- This led to the conclusion that atoms consist of small, dense positively charged nuclei surrounded by negatively charged electrons

An atom: a small positive nucleus, surrounded by negative electrons
- (Note: The atom is around 100,000 times larger than the nucleus!)
Worked example
In an α-particle scattering experiment, a student set up the apparatus below to determine the number of α-particles, n, incident per unit time on a detector held at various angles θ.

Which of the following graphs best represents the variation of n with θ from 0 to 90°?
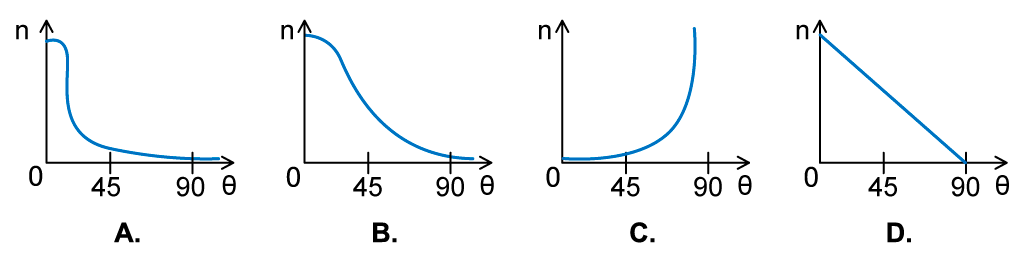
Answer: A
- The Rutherford scattering experience directed parallel beams of α-particles at gold foil
- The observations were:
- Most of the α-particles went straight through the foil
- The largest value of n will therefore be at small angles
- Some of the α-particles were deflected through small angles
- n drops quickly with increasing angle of deflection θ
- These observations fit with graph A

