Syllabus Edition
First teaching 2024
First exams 2026
Ozone Depletion (HL) (DP IB Environmental Systems & Societies (ESS)): Revision Note
Ozone depletion
Ozone depletion refers to the gradual thinning of the ozone layer in the Earth's stratosphere
This is due to human-made chemicals called ozone-depleting substances (ODSs)
These ODSs are released from products such as refrigerants, aerosols, and solvents
One of the most well known groups of ODSs are chlorofluorocarbons (CFCs)
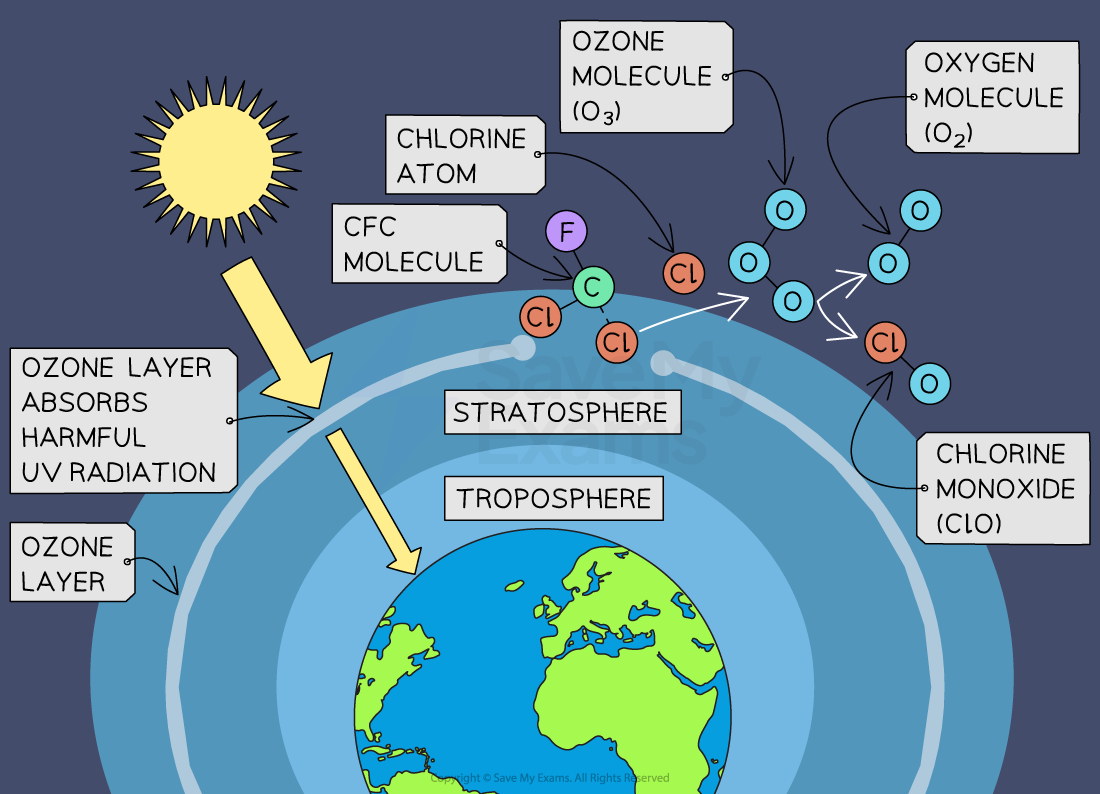
How CFCs cause ozone depletion
CFCs are stable compounds and do not readily degrade in the lower atmosphere (troposphere)
As CFCs are released into the atmosphere, they gradually drift upward into the stratosphere
CFCs absorb UV radiation in the stratosphere
This causes them to break down, releasing chlorine radicals
Stage 1:
A chlorine radical (Cl•) reacts with an ozone (O₃) molecule to form an oxygen (O₂) molecule and chlorine monoxide, which is also a chemical radical (ClO•)
Cl• + O3 → ClO• + O2
Stage 2:
The chlorine monoxide then combines with another ozone molecule to produce another chlorine radical and two oxygen molecules
ClO• + O3 → Cl• + 2O2
Overall reaction:
Overall, as a result of these two stages, ozone is converted to oxygen
2O3 → 3O2
As long as chlorine radicals are present in the stratosphere, ozone molecules will be continuously broken down through this cycle of reactions
The chlorine radicals are not used up in these reactions (they act as catalysts)
As a result, a single chlorine radical can destroy thousands of ozone molecules before being removed from the stratosphere

Unlock more, it's free!
Did this page help you?