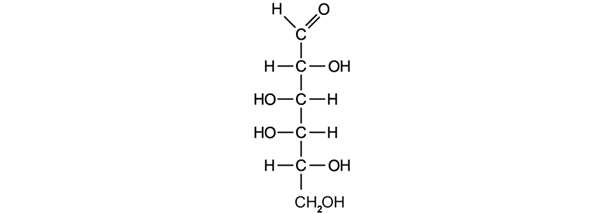
Urea, CO(NH2)2, is an animal waste product that can be used as a fertiliser. It can also be made artificially by reacting ammonia, NH3, with carbon dioxide, CO2, forming water as a co-product.
Formulate a balanced equation for the reaction.
Calculate the molar mass, M, of urea, CO(NH2)2.
Calculate the percentage of nitrogen in urea.
Give your answer to two decimal places.
The chemical structure of urea is shown below:

Deduce the total number of electron pairs in the molecule.
Did this page help you?