Oxidation States (DP IB Chemistry) : Revision Note
Oxidation States
Oxidation and reduction
There are three definitions of oxidation and reduction used in different branches of chemistry
Oxidation and reduction can be used to describe any of the following processes
Definitions and Examples of Oxidation & Reduction Table
Oxidation | Reduction |
|---|---|
Addition of oxygen e.g. 2Mg + O2 → MgO | Loss of oxygen e.g. 2CuO + C → 2Cu + CO2 |
Loss of hydrogen e.g. CH3OH | Gain of hydrogen e.g. C2H4 + H2 → C2H6 |
Loss of electrons e.g. Al → Al3+ + 3e– | Gain of electrons e.g. F2 + 2e– → 2F– |
Oxidation Number
The oxidation number or state of an atom is the charge that would exist on an individual atom if the bonding were completely ionic
It is like the electronic ‘status’ of an element
Oxidation numbers are used to...
tell if oxidation or reduction has taken place
work out what has been oxidised and/or reduced
construct half equations and balance redox equations
Atoms and simple ions
The oxidation number is the number of electrons which must be added or removed to become neutral
The oxidation number is always written with the charge before the number
Oxidation Number of Simple Ions Table
Atoms | Na in Na = 0 | Neutral already, no need to add any electrons |
|---|---|---|
Cations | Na in Na+ = +1 | Need to add 1 electron to make Na+ neutral |
Anions | Cl in Cl– = –1 | Need to take 1 electron away to make Cl– neutral |
Worked Example
What are the oxidation states of the elements in the following species?
C
Fe3+
Fe2+
O2-
He
Al3+
Answers:
0
+3
+2
-2
0
+3
So, in simple ions, the oxidation number of the atom is the charge on the ion:
Na+, K+, H+ all have an oxidation number of +1
Mg2+, Ca2+, Pb2+ all have an oxidation number of +2
Cl-, Br-, I- all have an oxidation number of -1
O2-, S2- all have an oxidation number of -2
Molecules or Compounds
In molecules or compounds, the sum of the oxidation numbers on the atoms is zero
Oxidation Number in Molecules or Compounds Table
Elements | H in H2 = 0 | Both are the same and must add up to zero |
|---|---|---|
Compounds | C in CO2 = +4 | 1 x (+4) and 2 x (–2) = 0 |
O in CO2 = –2 |
Since CO2 is a neutral molecule, the sum of the oxidation states must be zero
For this, one element must have a positive oxidation number and the other must be negative
How do you determine which is the positive one?
The more electronegative species will have the negative value
Electronegativity increases across a period and decreases down a group
O is further to the right than C in the periodic table so it has the negative value
How do you determine the value of an element's oxidation state?
From its position in the periodic table and/or
The other element(s) present in the formula
Many atoms, such as S, N and Cl can exist in a variety of oxidation states
The oxidation number of these atoms can be calculated by assuming that the oxidation number of the other atom is fixed
Here are six rules to deduce the oxidation number of an element
Oxidation Number Rules Table
Rule | Example |
|---|---|
1. The oxidation number of any uncombined element is zero | H2 Zn O2 |
2. Many atoms or ions have fixed oxidation numbers in compounds | Group 1 elements are always +1 Group 2 elements are always +2 Fluorine is always –1 Hydrogen is +1 (except for in metal hydrides like NaH, where it is –1) Oxygen is -2 (except in peroxides, where it is -1 and in F2O where it is +2) |
3. The oxidation number of an element in a mono-atomic ion is always the same as the charge | Zn2+ = +2 Fe3+ = +3 Cl– = –1 |
4. The sum of the oxidation number in a compound is zero | NaCl Na = +1 Cl = –1 Sum of oxidation numbers = 0 |
5. The sum of oxidation numbers in an ion is equal to the charge on the ion | SO42– S = +6 Four O atoms = 4 x (–2) Sum of oxidation numbers = –2 |
6. In either a compound or an ion, the more electronegative element is given the negative oxidation number | F2O Both F atoms = 2 x (–1) O = +2 Sum of oxidation numbers = 0 |
Worked Example
State the oxidation number of the atoms in blue in these compounds or ions.
a) P2O5
b) SO42-
c) H2S
d) Al2Cl6
e) NH3
f) ClO2-
Answer:
P2O5 | 5 O atoms = 5 x (–2) = –10 Overall charge compound = 0 2 P atoms = +10 P = +5 |
SO42- | 4 O atoms = 4 x (–2) = –8 Overall charge compound = –2 S = +6 |
H2S | 2 H atoms = 2 x (+1) = +2 Overall charge compound = 0 S = –2 |
Al2Cl6 | 6 Cl atoms = 6 x (–1) = –6 Overall charge compound = 0 2 Al atoms = +6 Al = +3 |
NH3 | 3 H atoms = 3 x (+1) = +3 Overall charge compound = 0 N = –3 |
ClO2- | 2 O atoms = 2 x (–2) = –4 Overall charge compound = –1 Cl = +3 |
Are oxidation numbers always whole numbers?
The answer is yes and no
When you try and work out the oxidation of sulfur in the tetrathionate ion S4O62- you get an interesting result!
Oxidation number of S in S4O62–
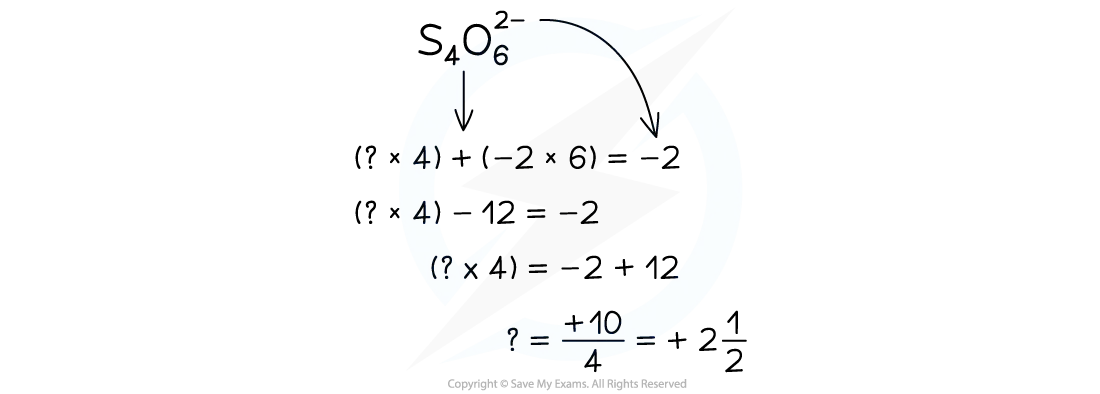
The oxidation number of sulfur in S4O62- is a fraction
The fact that the oxidation number comes out to +2.5 does not mean it is possible to get half an oxidation number
This is only a mathematical consequence of four sulfur atoms sharing +10 oxidation number
The four sulfur atoms are in two different environments and the +2.5 is showing the average oxidation number of these two environments
Single atoms can only have an integer oxidation number, because you cannot have half an electron!
Examiner Tips and Tricks
Oxidation number and oxidation state are often used interchangeably, though IUPAC does not distinguish between the two terms
Oxidation numbers are represented by Roman numerals according to IUPAC
Naming Transition Metal Compounds
Transition metals are characterized by having variable oxidation numbers.
Oxidation numbers can be used in the names of compounds to indicate which oxidation number a particular element in the compound is in
Where the element has a variable oxidation number, the number is written afterwards in Roman numerals.
This is called the STOCK NOTATION (after the German inorganic chemist Alfred Stock), but is not widely used for non-metals, so SO2 is sulphur dioxide rather than sulphur(IV) oxide
For example, iron can be both +2 and +3 so Roman numerals are used to distinguish between them
Fe2+ in FeO can be written as iron(II) oxide
Fe3+ in Fe2O3 can be written as iron(III) oxide
Worked Example
Name these transition metal compounds.
Cu2O
MnSO4
Na2CrO4
KMnO4
Na2Cr2O7
Answers:
Copper(I) oxide
The oxidation number of 1 O atom is -2
Cu2O has overall no charge
So, the oxidation number of Cu is +1
Manganese(II) sulfate
The charge on the sulfate ion is -2
So, the charge on Mn and oxidation number is +2
Sodium chromate(VI)
The oxidation number of 2 Na atoms is +2
Therefore, CrO4 has an overall -2 charge
So, the oxidation number of Cr is +6
Potassium manganate(VII)
The oxidation number of a K atom is +1
Therefore, MnO4 has an overall -1 charge
So, the oxidation number of Mn is +7
Sodium dichromate(VI)
The oxidation number of 2 Na atoms is +2
Therefore, Cr2O7 has an overall -2 charge
So the oxidation number of Cr is +6
To distinguish it from CrO4 we use the prefix di in front of the anion
Examiner Tips and Tricks
The answer to question 2 should strictly speaking be manganese (II) sulfate(VI) since sulfur is an element with a variable oxidation number
However, the sulfate ion is a common ion whose name and formula you should know and you are only required to name transition metal compounds using Stock Notation

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
