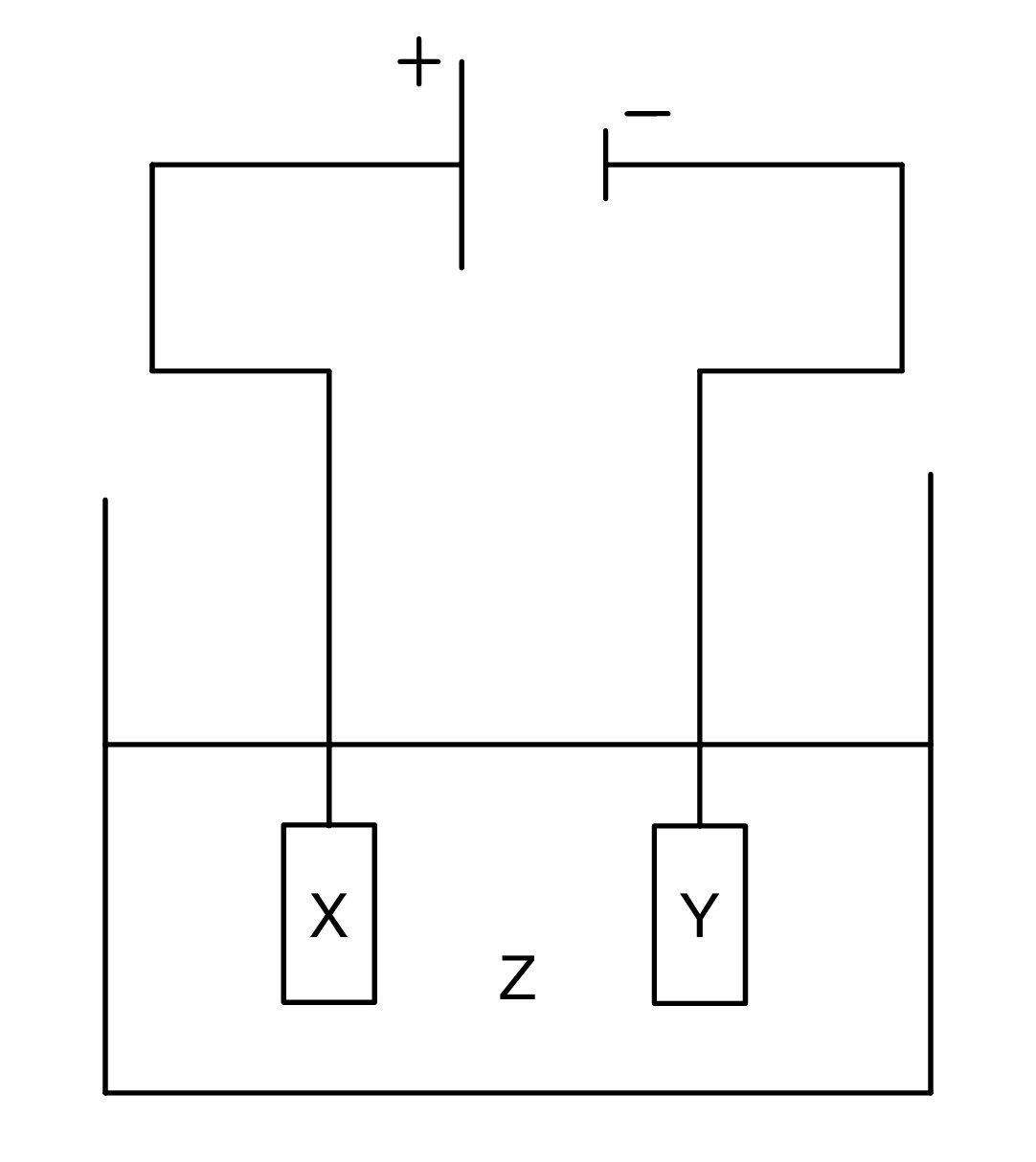
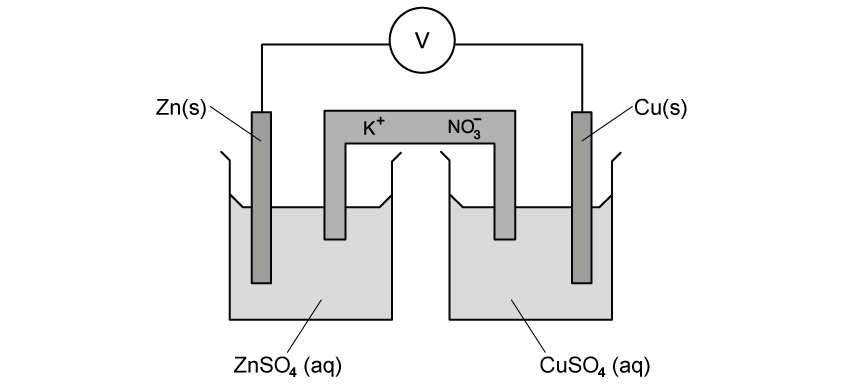
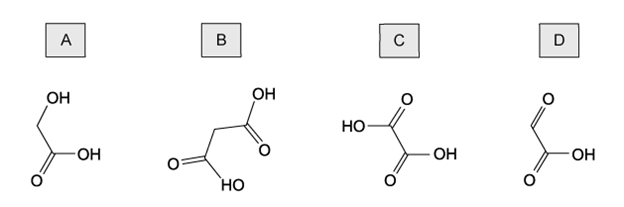
Which row correctly describes oxidation and reduction in terms of the transfer of electrons and changes in oxidation state?
| Transfer of electrons | Change in oxidation state | ||
| oxidation | reduction | oxidation | reduction |
A | gain | loss | increase | decrease |
B | loss | gain | increase | decrease |
C | loss | gain | decrease | increase |
D | gain | loss | decrease | increase |
Did this page help you?