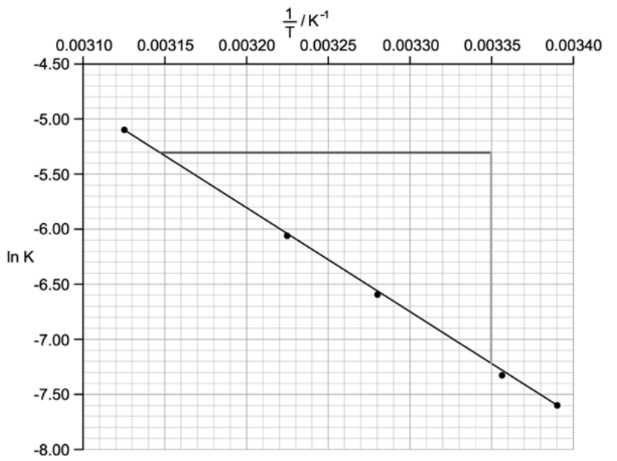
A student performs two reactions and measures the rate of product formation.
Reaction 1: 1.5g of solid calcium carbonate is added to 100 cm3 of 0.5 M hydrochloric acid
Reaction 2: 100 cm3 of distilled water is then added to 100 cm3 of 0.5 M hydrochloric acid then 1.5g of solid calcium carbonate is added
The rate of reaction 1 was faster than the rate of reaction 2.
Which of the following 3 hypotheses correctly describes the difference in the rate?
I. Adding water reduces the frequency of collisions between reactant molecules.
II. Adding water reduces the proportion of effective collisions between reactant molecules.
III. Adding water reduces the proportion of reactant molecules possessing the activation energy.


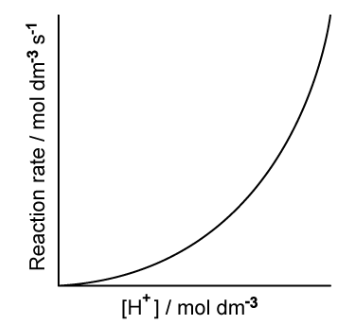
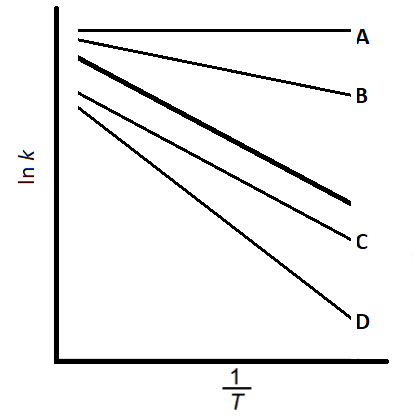
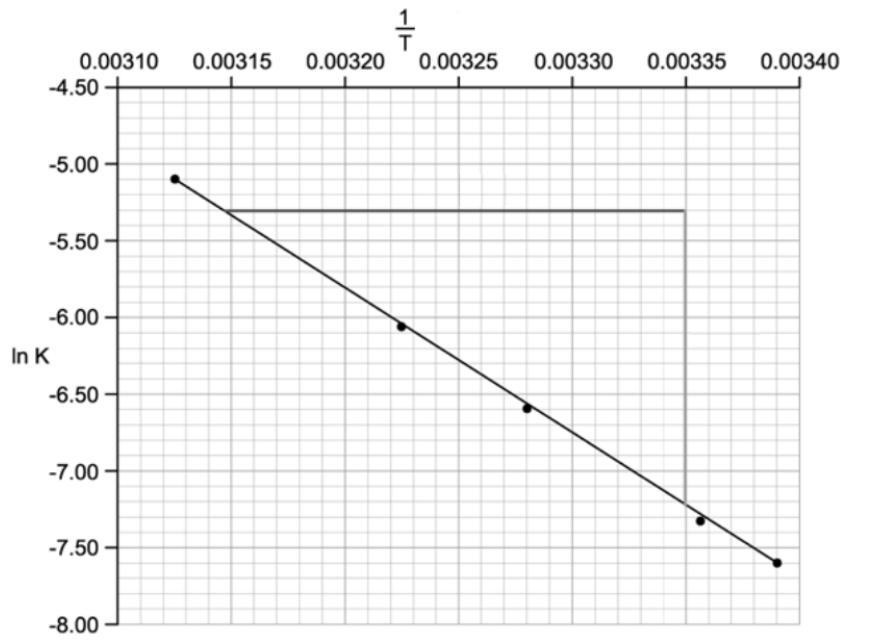
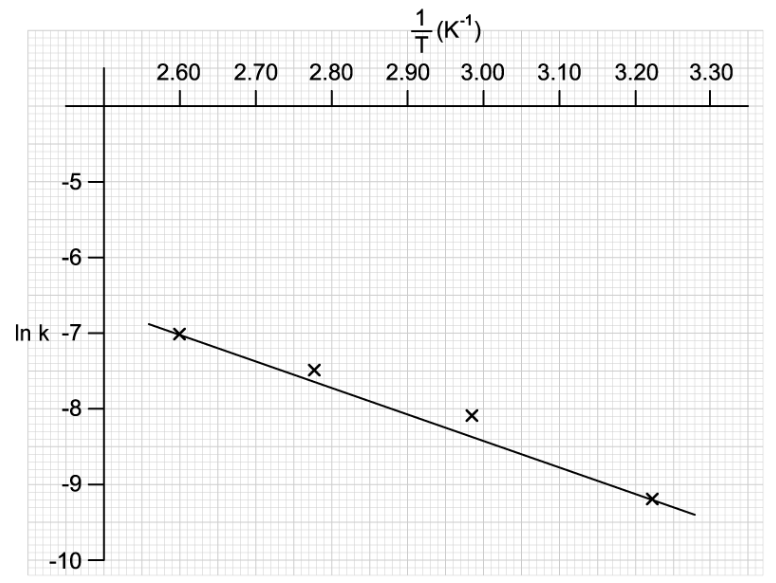








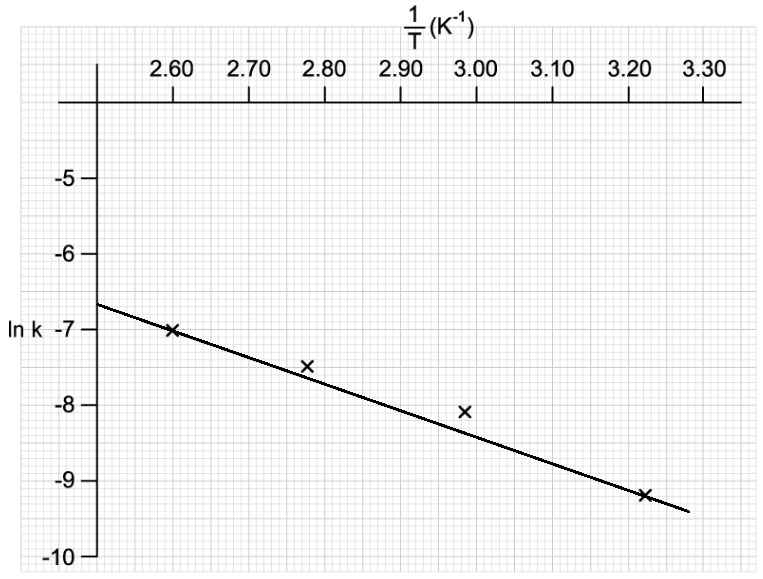









![Graph showing a curve increasing exponentially. X-axis: [H⁺] concentration in mol dm⁻³. Y-axis: Reaction rate in mol dm⁻³ s⁻¹.](https://cdn.savemyexams.com/cdn-cgi/image/f=auto,width=3840/https://cdn.savemyexams.com/uploads/2025/09/57162_httpscdn-savemyexams-comuploads202206conc-v-rate-graph-.png)