Reducing Errors
- Random errors can be reduced by repeated trials and measurements
- Multiple measurements when averaged will reduce the impact of a random error on the average
- The more readings you have the lower the possibility that a random error will skew the results
- If you spot a random error in a data table then you can omit it in the calculation of an average
- For example, in a titration you can leave out results that are not concordant when finding the average titre:
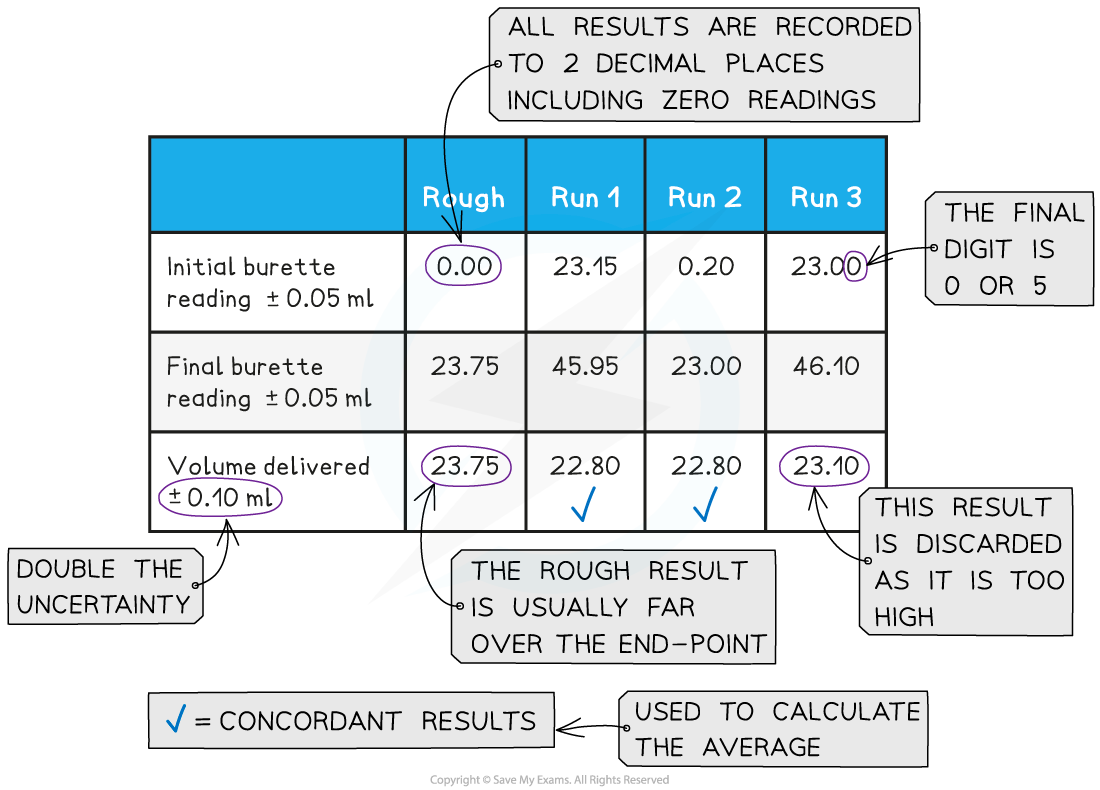
Calculating the average volume delivered in a titration should not include non-concordant volumes. Run 3 (and the Rough run) is omitted from the calculation of the average volume delivered
- Systematic errors cannot be reduced by repetition
- Systematic errors can only be reduced by changing the procedure and making sure you are using the instruments correctly
- If you cannot actively reduce systematic errors you must still try to identify them and comment on them in your evaluation



