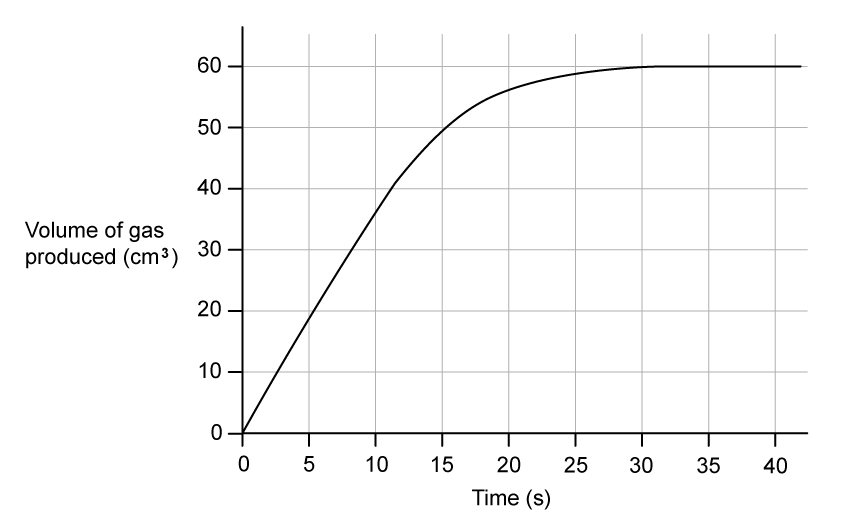
A group of students planned how to investigate the effect of changing the concentration of H2SO4 on the initial rate of reaction with magnesium:
Mg (s) + H2SO4 (aq) format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22math12bba9f4124283edd644799e0ce%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%2210.5%22%20y%3D%2216%22%3E%26%23x2192%3B%3C%2Ftext%3E%3C%2Fsvg%3E) MgSO4 (aq) + H2 (g)
MgSO4 (aq) + H2 (g)
They decided to measure how long the reaction took to complete when similar masses of magnesium were added to acid.
Two methods were suggested:
Method 1 - Use small pieces of magnesium ribbon, an excess of acid and record the time taken for the magnesium ribbon to disappear
Method 2 - Use large strips of magnesium ribbon, an excess of magnesium and record the time taken for bubbles to stop forming
Deduce, giving a reason, which of method 1 and method 2 would be the least affected if the masses of magnesium ribbon used varied slightly between each experiment.