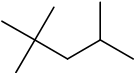
State the difference between complete and incomplete combustion of a hydrocarbon fuel, such as octane, including the products formed.
Write a balanced symbol equation for the complete combustion of decane, C10H22.
Decane is a major component of diesel fuel.
i) Write a balanced symbol equation for the incomplete combustion of decane, C10H22, forming carbon as one product.
[1]
ii) What might you observe to tell you that carbon is being formed?
[1]
Did this page help you?