Expansion of the Octet (HL) (DP IB Chemistry) : Revision Note
Expansion of the Octet
Expansion of the octet
Elements in period 3 and above have the possibility of having more than eight electrons in their valence shell
This is because there is a d-subshell present which can accommodate additional pairs of electrons
This is known as the expansion of the octet
The concept explains why structures such as PCl5 and SF6 exist, which have 5 and 6 bonding pairs of electrons respectively, around the central atom
Five electron pairs
Phosphorus pentachloride, PCl5
An example of a molecule with five bonding electron pairs is phosphorus pentachloride, PCl5
The total number of valence electrons is = P + 5Cl = 5 + (5 x 7) = 40
The number of bonding pairs is 5, which accounts for 10 electrons
The remaining 30 electrons would be 15 lone pairs, so that each Cl has 3 lone pairs
The completed Lewis formula looks like this:
Lewis formula of PCl5

The octet of the central phosphorous atom has been expanded to hold 10 electrons
Sulfur tetrafluoride, SF4
The total number of valence electrons is = S + 4F = 6 + (4 x 7) = 34
The number of bonding pairs is 4, which accounts for 8 electrons
The remaining 26 electrons would be 13 lone pairs
Fluorine cannot expand the octet so each fluorine would accommodate 3 lone pairs, accounting for 24 electrons, leaving 1 lone pair on the sulfur (sulfur has expanded the octet)
The completed Lewis formula looks like this:
Lewis formula of SF4

The octet of the central sulfur atom has been expanded to hold 10 electrons
Chlorine trifluoride, ClF3
The total number of valence electrons is = Cl + 3F = 7 + (3 x 7) = 28
The number of bonding pairs is 3, which accounts for 6 electrons
The remaining 22 electrons would be 11 lone pairs
Fluorine cannot expand the octet so each fluorine would accommodate 3 lone pairs, accounting for 18 electrons, leaving 2 lone pairs on the chlorine
The completed Lewis formula looks like this:
Lewis formula of ClF3

The octet of the central chlorine atom has been expanded to hold 10 electrons
Triiodide ion, l3-
The total number of valence electrons is = 3I + the negative charge = (3 x 7) + 1 = 22
The number of bonding pairs is 2, which accounts for 4 electrons
The remaining 18 electrons would be 9 lone pairs
Iodine would accommodate 3 lone pairs, accounting for 12 electrons, leaving 3 lone pairs on the central iodine
The completed Lewis formula looks like this:
Lewis formula of I3–

The octet of the central iodine atom has been expanded to hold 10 electrons
Six electron pairs
Sulfur hexafluoride, SF6
An example of a molecule with six bonding electron pairs is sulfur hexafluoride, SF6
The total number of valence electrons is = S + 6F = 6 + (6 x 7) = 48
The number of bonding pairs is 6, which accounts for 12 electrons
The remaining 36 electrons would be 18 lone pairs, so that each F has 3 lone pairs, accounting for all electrons and no lone pairs
The completed Lewis formula looks like this:
Lewis formula of SF6

The octet of the central sulfur atom has been expanded to hold 12 electrons
Bromine pentafluoride, BrF5
The total number of valence electrons is = Br + 5F = 7 + (5 x 7) = 42
The number of bonding pairs is 5, which accounts for 10 electrons
The remaining 32 electrons would be 16 lone pairs
Fluorine cannot expand the octet so each fluorine would accommodate 3 lone pairs, accounting for 30 electrons, leaving 1 lone pair on the bromine
The completed Lewis formula looks like this:
Lewis formula of BrF5

The octet of the central bromine atom has been expanded to hold 12 electrons
Xenon tetrafluoride, XeF4
The total number of valence electrons is = Xe + 4F = 8 + (4 x 7) = 36
The number of bonding pairs is 4, which accounts for 8 electrons
The remaining 28 electrons would be 14 lone pairs
Each fluorine would accommodate 3 lone pairs, accounting for 24 electrons, leaving 2 lone pairs on the xenon
The completed Lewis formula looks like this:
Lewis formula of XeF4

The octet of the central xenon atom has been expanded to hold 12 electrons
Revisiting Valence Shell Electron Pair Repulsion Theory (VSEPR)
Molecular shapes and the angles between bonds can be predicted using the three basic rules associated with valence shell electron pair repulsion theory known by the abbreviation VSEPR theory
VSEPR theory consists of three basic rules:
All electron pairs and all lone pairs arrange themselves as far apart in space as possible.
Lone pairs repel more strongly than bonding pairs
Multiple bonds behave like single bonds
For more information about valence shell electron pair repulsion theory, see our revision note on Shapes of Molecules
Molecular geometry versus electron domain geometry
It is important to distinguish between molecular geometry and electron domain geometry in exam questions
Molecular geometry refers to the shape of the molecules based on the relative orientation of the atoms
Electron domain geometry refers to the relative orientation of all the bonding and lone pairs of electrons
The Lewis formula for water enables us to see that there are four electron pairs around the oxygen so the electron domain geometry is tetrahedral
However, the molecular geometry shows us there are two angled bonds so the shape is bent, angular, bent linear or V-shaped (when viewed upside down)
Lewis formula and molecular shape of water

The Lewis formula of water and molecular shape can be used to determine the electron domain and molecular geometries
Five electron domains
Table of molecular geometries associated with five electron domains
Electron domain geometry | Bonding pairs | Lone pairs | Molecular geometry | Shape example |
|---|---|---|---|---|
trigonal bipyramidal | 5 | 0 | trigonal bipyramidal |  |
trigonal bipyramidal | 4 | 1 | seesaw | 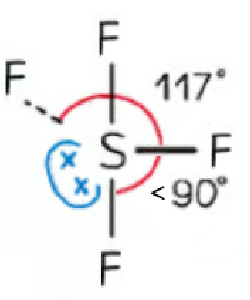 |
trigonal bipyramidal | 3 | 2 | T-shape | 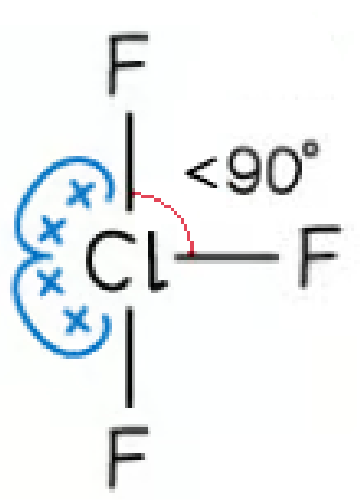 |
trigonal bipyramidal | 2 | 3 | Linear |  |
PCl5 is a symmetrical molecule so the electron cloud charge is evenly spread
This means that it will be a non-polar molecule as any dipoles from the P–Cl bonds would be cancelled out
SF4 and ClF3 are asymmetrical molecules having one or two lone pairs on one side of the central axis making the overall molecule polar
Six electron domains
Table of molecular geometries associated with six electron domains
Electron domain geometry | Bonding pairs | Lone pairs | Molecular geometry | Shape example |
|---|---|---|---|---|
octahedral | 6 | 0 | octahedral |  |
octahedral | 5 | 1 | square based pyramid |  |
octahedral | 4 | 2 | square planar |  |
SF6 is a symmetrical molecule so the electron cloud charge is evenly spread with 90o between the bonds
This means that it will be a non-polar molecule as any dipoles from the S–F bonds would be cancelled out
XeF4 is also non-polar despite having two lone pairs
The bonding pairs are at 90o to the plane and the lone pairs are at 180o
The lone pairs are arranged above and below the square plane resulting in an even distribution of electron cloud charge
BrF5 is asymmetrical having a lone pair at the base of the pyramid making the overall molecule polar
Worked Example
What is the electron domain geometry, molecular geometry and F-Xe-F bond angle of xenon difluoride, XeF2?
Answer
Count the valence electrons = Xe + 2F = 8 + (2 x 7) = 22
There are two bonding pairs, accounting for 4 electrons, so 18 electrons remain
Each fluorine should have 3 lone pairs, accounting for 6 pairs or 12 electrons, which leaves 3 lone pairs on the xenon
Xenon, therefore, has 2 bonding pairs and 3 lone pairs meaning it has:
Electron domain geometry = trigonal bipyramid
Molecular geometry = linear
The bond angle will be 180o (having the same structure as the triiodide ion)
Examiner Tips and Tricks
Lewis structure and Lewis formula may be used interchangeably, but Lewis formula is the preferred term in the specification.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
