Nucleophilic Substitution in Halogenoalkanes (HL) (DP IB Chemistry): Revision Note
Nucleophilic substitution in halogenoalkanes
In nucleophilic substitution, the halogen atom in a halogenoalkane is replaced by a nucleophile
A nucleophile is more reactive if it can readily donate a lone pair of electrons
The hydroxide ion, OH-, is a stronger nucleophile than water because:
It has a full negative charge
This means that it has a readily available lone pair of electrons
Water (H2O) is a neutral molecule with partial charges, δ+ and δ-
Its lone pairs are less available for reaction
However, it can still act as a nucleophile
This contrast is shown in the Lewis structures of OH- and H2O:

Examiner Tips and Tricks
In general:
Negatively charged species are stronger nucleophiles than neutral molecules
A conjugate base is a stronger nucleophile than its conjugate acid
e.g. OH- is stronger nucleophile than H2O
There are two types of nucleophilic substitution reaction:
SN1
SN2
The type of nucleophilic substitution depends on the halogenoalkane involved
SN1 reactions
In tertiary halogenoalkanes, the carbon that is attached to the halogen is also bonded to three alkyl groups
These halogenoalkanes undergo nucleophilic substitution by an SN1 mechanism
‘S’ stands for ‘substitution’
‘N’ stands for ‘nucleophilic’
‘1’ means that the rate of the reaction
This is determined by the slowest step of the reaction
It depends on the concentration of one reagent - the halogenoalkane

SN1 mechanism
The SN1 mechanism is a two-step reaction
In the first step:
The C-X bond breaks heterolytically
The halogen leaves the halogenoalkane as an X- ion
This is the slow, rate-determining step
A tertiary carbocation intermediate is formed
In the second step:
The nucleophile attacks the positively charged carbon in the tertiary carbocation intermediate
This two-step process is evident in the energy profile diagram for an SN1 reaction:

The rate-determining step depends only on the concentration of the halogenoalkane
Therefore, the rate equation for an SN1 reaction is:
rate = k[halogenoalkane]
In terms of molecularity, an SN1 reaction is unimolecular
Example of SN1 mechanism
The nucleophilic substitution of 2-bromo-2-methylpropane by hydroxide ions is SN1
The product is 2-methyl-2-propanol

Examiner Tips and Tricks
In SN1 mechanisms, heterolytic fission occurs when a bond breaks and both bonding electrons go to one atom
This produces a cation and an anion
The movement of the electron pair is shown using a double-headed arrow (→)
In other organic mechanisms (e.g. some radical reactions), homolytic fission occurs instead
Each atom takes one electron from the bond
This produces two free radicals
Homolytic fission is shown using a single-headed arrow (
)
SN2 reactions
In primary halogenoalkanes, the carbon that is attached to the halogen is bonded to one alkyl group
These halogenoalkanes undergo nucleophilic substitution by an SN2 mechanism
‘S’ stands for ‘substitution’
‘N’ stands for ‘nucleophilic’
‘2’ means that the rate of the reaction
This is determined by the slowest step of the reaction
It depends on the concentration of two reagents - the halogenoalkane and the nucleophile

SN2 mechanism
The SN2 mechanism is a one-step reaction
The nucleophile attacks the δ⁺ carbon atom of the halogenoalkane from the opposite side of the leaving group
This forms a new bond between the nucleophile and the carbon atom
At the same time, the C–X bond breaks by heterolytic fission
The halogen leaves as an X⁻ ion
The nucleophile donates a lone pair of electrons to form the new C–Nu bond
The halogen takes both bonding electrons as it leaves
All of these changes happen simultaneously in a one-step process
No intermediate is formed
The energy profile for an SN2 reaction shows a single peak representing the transition state:

As this is a one-step reaction, the rate-determining step depends on the concentrations of the halogenoalkane and nucleophile
Therefore, the rate equation for an SN2 reaction is:
rate = k[halogenoalkane][nucleophile]
In terms of molecularity, an SN2 reaction is bimolecular
Example of SN2 mechanism
The nucleophilic substitution of bromoethane by hydroxide ions is SN2
The product is ethanol

Inversion of configuration in SN2
In SN2 reactions, the nucleophile always attacks from the opposite side of the leaving group due to steric hindrance
An attack from the same side as the bromine atom is a frontal attack
An attack from the opposite side to the bromine atom is a backside or rear-side attack
In bromoethane, the large bromine atom blocks a frontal attack
As a result, the hydroxide ion performs a rear-side (backside) attack, rather than a frontal one
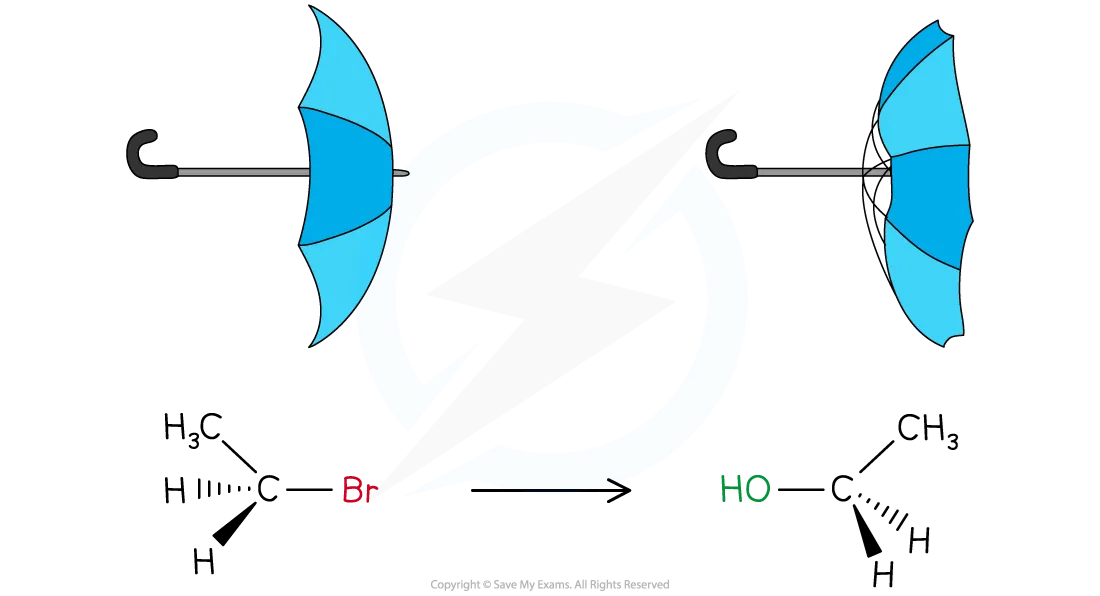
This causes the molecule to undergo inversion of configuration
This is when the three other groups around the carbon are flipped
A common analogy is an umbrella turning inside out in the wind
As the new C–OH bond forms, the C–Br bond breaks causing the bromine to leave as a bromide ion, Br⁻
Examiner Tips and Tricks
When explaining SN2 mechanisms involving inversion of configuration, you must clearly:
Use partial charges, δ+ and δ-, to show polarity of bonds and electron movement
Show wedge, dashed, and solid bonds to illustrate the 3D structure before and after inversion
Draw the transition state with:
A dotted bond between the carbon and the incoming nucleophile
A dotted bond to the leaving group (halogen)
Remember: the transition state is not an intermediate
It is a temporary high-energy arrangement with partial bonds

Unlock more, it's free!
Did this page help you?