Rate Equation (HL) (DP IB Chemistry): Revision Note
Rate equation
The rate of reaction can be found by measuring the:
Decrease in reactant concentration over time
Increase in product concentration over time
The units for rate of reaction are mol dm-3 s-1
Rate of reaction
The following general reaction will be used as an example to study the rate of reaction
D (aq) → E (aq) + F (g)
The rate of reaction at different concentrations of D is measured and tabulated
[D] (mol dm-3) | Rate (mol dm-3 s-1) | gradient = |
|---|---|---|
3.00 | 2.00 x 10-3 | 6.67 x 10-4 |
2.00 | 1.33 x 10-3 | 6.67 x 10-4 |
1.00 | 6.67 x 10-4 | 6.67 x 10-4 |
Plotting the results on a graph shows a directly proportional relationship between the rate of reaction and concentration of D:
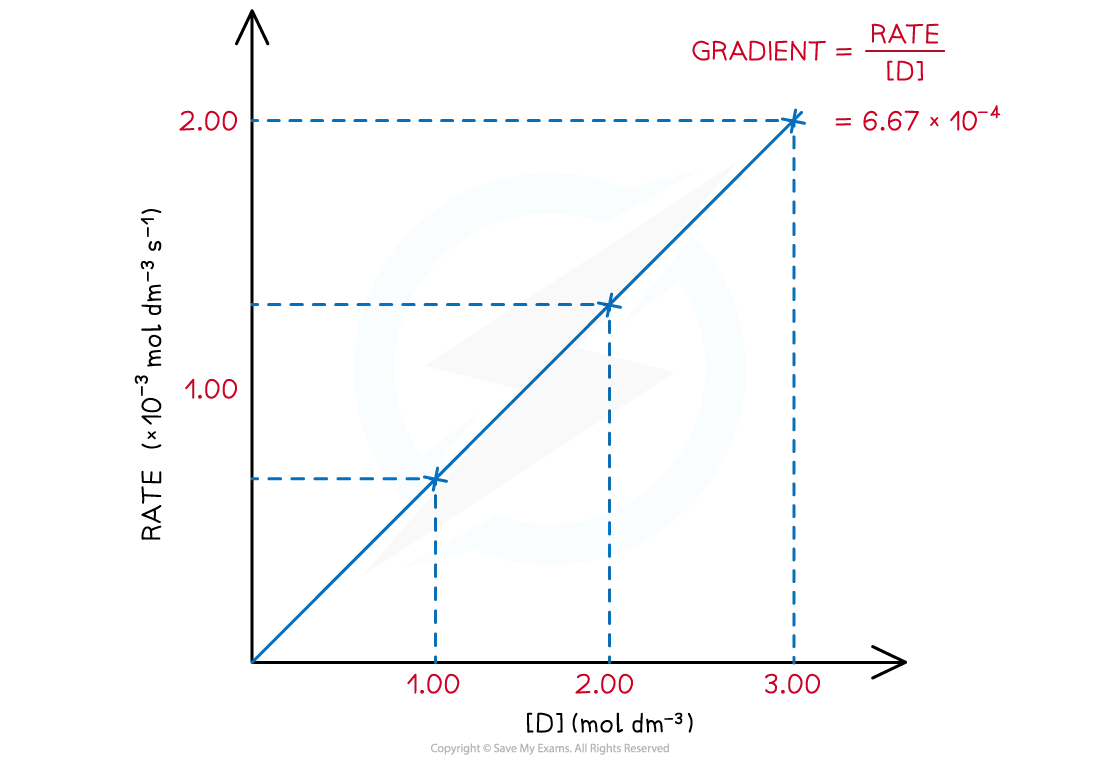
The value of the gradient is constant as it is a straight line
The gradient at each concentration is calculated by:
gradient =
This leads to the rate expression:
Rate ∝ [D] or Rate = k[D]
This rate expression means that:
If the concentration of D doubles, then the rate doubles
If the concentration of D halves, then the rate halves
Not all reactions show this directly proportional relationship between rate and reactant concentration
The rate equation and orders of reaction help explain how rate is affected by each reactant
What is the rate equation?
The rate equation expresses the relationship between reaction rate and species concentrations, usually in the form:
Rate = k [species 1] m [species 2]n
In most reactions:
These species are reactants
But catalysts or products may also appear
Intermediates do not appear
The following reaction will be used to discuss rate equations:
A (aq) + B (aq) → C (aq) + D (g)
The rate equation for this reaction is:
Rate of reaction = k [A]m [B]n
Where:
[A] and [B] are the concentrations of the reactants
m and n are orders with respect to each reactant
Rate equations depend on the reaction mechanism
They must be determined experimentally
They cannot be found from the stoichiometric equations
What is the order of reaction?
The order of reaction shows how the rate of a reaction depends on the concentration of a reactant
It is the power to which the concentration is raised in the rate equation
The order can be positive, negative, or fractional
Fractional orders suggest that the reaction involves multiple steps
Zero order
When the order of reaction with respect to a reactant is 0:
Changing the concentration has no effect on the rate of reaction
The reactant does not appear in the rate equation
First order
When the order of reaction with respect to a reactant is 1:
The rate is directly proportional to the concentration
Doubling the concentration doubles the rate
The reactant is included in the rate equation (no power shown)
Second order
When the order of reaction with respect to a reactant is 2:
The rate is proportional to the square of the concentration
Doubling the concentration quadruples the rate
The reactant is included in the rate equation, squared
Overall order
The overall order of reaction is the sum of the powers of the reactants in a rate equation:
Rate of reaction = k [A]m [B]n
Overall order of reaction = m + n
Examiner Tips and Tricks
You may be shown reactions involving fractional orders such as:
Ethanal decomposition:
Rate = k[CH3CHO]3/2
Hydrogen reacting with bromine:
Rate = k[H2][Br2]1/2
Carbon monoxide reacting with chlorine:
Rate = k[CO]2[Cl2]1/2
You are not expected to calculate with fractional orders in exams
For calculations, only values of 0, 1 or 2 need to be considered
Worked Example
The chemical equation for the thermal decomposition of dinitrogen pentoxide is:
2N2O5 (g) → 4NO2 (g) + O2 (g)
The rate equation for this reaction is:
Rate = k[N2O5 (g)]
State the order of the reaction with respect to dinitrogen pentoxide.
Deduce the effect on the rate of reaction if the concentration of dinitrogen pentoxide is tripled.
Answers:
The order with respect to dinitrogen pentoxide is 1 / first order
N2O5 appears in the rate equation
Its concentration is raised to the power of 1
Therefore, the order with respect to N2O5 is first order
If the concentration of dinitrogen pentoxide is tripled, the rate of reaction triples
The reaction is first order
Rate is directly proportional to the concentration of dinitrogen pentoxide
Any change to the concentration of the dinitrogen pentoxide causes the same change to the rate of reaction
Worked Example
The following equation represents the oxidation of bromide ions in acidic solution:
BrO3- (aq) + 5Br- (aq) + 6H+ (aq) → 3Br2 (l) + 3H2O (l)
The rate equation for this reaction is:
Rate = k[BrO3- (aq)][Br- (aq)][H+ (aq)]
State the overall order of the reaction.
Deduce the effect on the rate of reaction if the concentration of bromate ions is doubled and the concentration of bromide ions is halved.
Answers:
The overall order of the reaction 3 / third order
Each reactant appears in the rate equation
Each reactant is raised to the power of 1
Therefore, each is first order
So, the overall order of the reaction is:
1 (BrO3-) + 1 (Br-) + 1 (H+) = 3
The rate of reaction does not change
The reaction is first order with respect to both BrO3- and Br-
If [BrO3-] is doubled, the rate doubles
If [Br-] is halved, the rate halves
These changes cancel out, so the overall rate stays the same
How can the rate equation be deduced from experimental data?
The following reaction will be used to determine the rate equation from experimental concentration and rate data:
(CH3)3CBr + OH- → (CH3)3COH + Br-
The table below shows the concentrations of reactants and the initial rates of reaction under different conditions:
Experiment | Initial [(CH3)3CBr] / | Initial [OH-] / | Initial rate of reaction / |
|---|---|---|---|
1 | 1.0 x 10-3 | 2.0 x 10-3 | 3.0 x 10-3 |
2 | 2.0 x 10-3 | 2.0 x 10-3 | 6.0 x 10-3 |
3 | 1.0 x 10-3 | 4.0 x 10-3 | 1.2 x 10-2 |
4 | 1.5 x 10-3 | 4.0 x 10-3 | 4.5 x 10-3 |
To write the rate equation:
Determine the order with respect to each reactant
This can be done by analysing the table of concentration and rate data
Use the reaction equation to write an initial rate equation
Apply the orders of reactant to the initial rate equation
How to determine order from data
Choose two experiments where only one reactant changes in concentration
Calculate how the concentration changes
Calculate how the rate changes
Use this information to deduce the order of reaction with respect to that reactant
Repeat for the other reactant(s)
Order with respect to [(CH3)3CBr]
In experiments 1 and 2:
[(CH3)3CBr] changes
[OH-] remains constant
The [(CH3)3CBr] doubles
Experiment 1 [(CH3)3CBr] = 1.0 x 10-3 mol dm-3
Experiment 2 [(CH3)3CBr] = 2.0 x 10-3 mol dm-3
The rate of the reaction doubles
Experiment 1 rate = 3.0 x 10-3 mol dm-3 s-1
Experiment 2 rate = 6.0 x 10-3 mol dm-3 s-1
The order with respect to [(CH3)3CBr] is 1 / first order
[Change in concentration]order = change in rate
[2]order = 2
[2]1 = 2
Order with respect to [OH–]
In experiments 1 and 3:
[OH–] changes
[(CH3)3CBr] remains constant
The [OH–] doubles
Experiment 1 [OH–] = 2.0 x 10-3 mol dm-3
Experiment 3 [OH–] = 4.0 x 10-3 mol dm-3
The rate of the reaction increases by a factor of 4
Experiment 1 rate = 3.0 x 10-3 mol dm-3 s-1
Experiment 3 rate = 1.2 x 10-2 mol dm-3 s-1
The order with respect to [OH–] is 2 / second order
[Change in concentration]order = change in rate
[2]order = 4
[2]2 = 4
Building the rate equation
Now that both orders of reaction are known, we can write the full rate equation
The initial rate equation is:
Rate = k [(CH3)3CBr](m) [OH–](n)
The order with respect to [(CH3)3CBr] is 1 / first order
First order reactants are written as [X]
The order with respect to [OH–] is 2 / second order
Second order reactants are written as [X]²
This gives the final rate equation:
Rate = k [(CH3)3CBr] [OH–]2
The reaction is:
First order with respect to (CH3)3CBr
Second order with respect to OH-
Third order overall
Examiner Tips and Tricks
Examiners will often give concentration and rate data in standard form to test your mathematical skills!
Take your time because it is easy to make a mistake
The most common mistake is failing to notice a factor of ten, e.g. one rate value is x10-4 while the rest are x 10-3

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?