Energy Profiles With & Without Catalysts (DP IB Chemistry) : Revision Note
Energy Profiles With & Without Catalysts
How do catalysts increase the rate of reaction?
A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation energy than the uncatalysed reaction
The catalyst remains chemically unaltered by the end of the reaction
How a catalyst increases the rate of reaction

The diagram shows that the catalyst speeds up a reaction that would normally be slow due to the high activation energy. The catalyst is not used up in the chemical reaction and is not taking part in the chemical reaction
Catalysts are important in reducing the environmental impact of industrial processes by:
Reducing the energy requirements of processes as they enable reactions to occur at lower temperatures and pressures
Reducing waste products as they can be reused and are only used in small quantities, increasing atom economy
Increasing the selectivity of processes, promoting specific reactions and suppressing undesired side reactions
Catalysts can be divided into two types:
Homogeneous catalysts
Heterogeneous catalysts
Homogeneous means that the catalyst is in the same phase as the reactants
For example, the reactants and the catalysts are all liquids
Heterogeneous means that the catalyst is in a different phase to the reactants
For example, the reactants are gases but the catalyst used is a solid
Energy profiles of reactions with catalysts
The lower activation energy of the alternative pathway used by a catalyst can be shown on an energy profile
Energy profile with and without a catalyst

The diagram shows that the catalyst allows the reaction to take place through a different mechanism, which has a lower activation energy than the original reaction
Examples of catalysts
Enzymes are biological catalysts
Enzymes act as catalysts in biological systems, controlling many biochemical reactions within cells
As well as being important for controlling reactions in cells, they are also important in industry
Enzymes allow industrial reactions to happen at lower temperatures and pressures than usually needed, saving money and energy
Transition metals are often used as catalysts due to their ability to form more than one stable oxidation state
For more information about the uses of transition metals as catalysts required in Higher Level Chemistry, see our revision note on the Characteristic Properties of Transition Elements
Worked Example
The energy profile below shows the energy changes for a reaction with and without a catalyst.
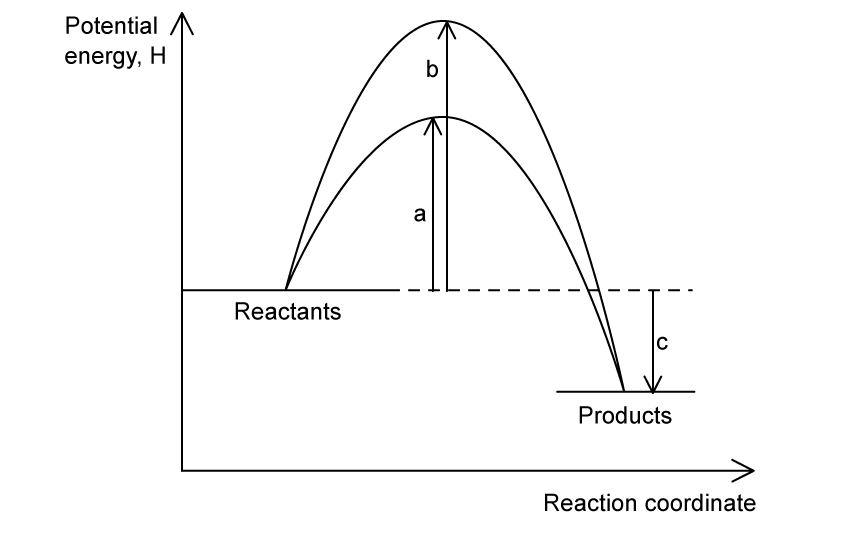
Which symbols represent the enthalpy change, ∆H, and the activation energy, Ea, for the reaction using a catalyst?
| ΔH | Ea (with catalyst) |
|---|---|---|
A. | a | c |
B. | b | c |
C. | c | a |
D. | b - a | c |
Answer:
The correct option is C.
By definition, the enthalpy change is the difference in energy content between reactants and products, in this case, arrow c
The catalyst lowers the activation energy, which corresponds to arrow a

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
