Levels of Protein Structure (DP IB Biology) : Revision Note
Primary Structure
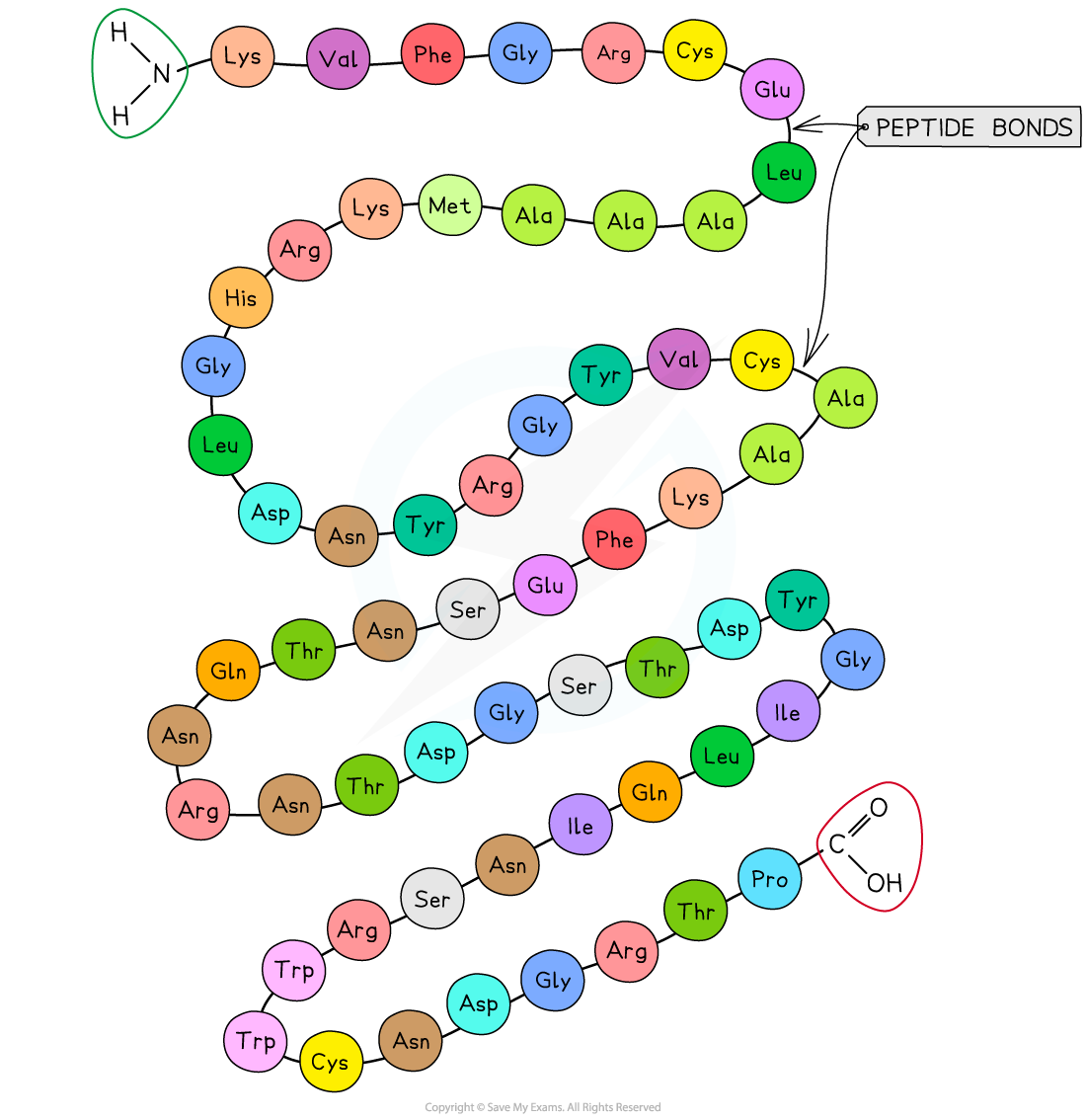
The sequence of amino acids makes up the primary structure of a protein
The DNA of a cell determines the primary structure of a protein by instructing the cell to add amino acids in a specific, ordered sequence
The precise position of each amino acid within the structure determines the eventual three-dimensional shape of proteins
The same sequence will always give rise to the same three-dimensional shape, meaning that proteins have precise, predictable and repeatable structures
Examiner Tips and Tricks
Make sure that you refer to the 'sequence' or 'order' of amino acids when describing primary structure, as just referring to a 'chain' of amino acids will not be enough here. It is the exact position of each amino acid within the chain that will determine the upper levels of structure.
Secondary Structure
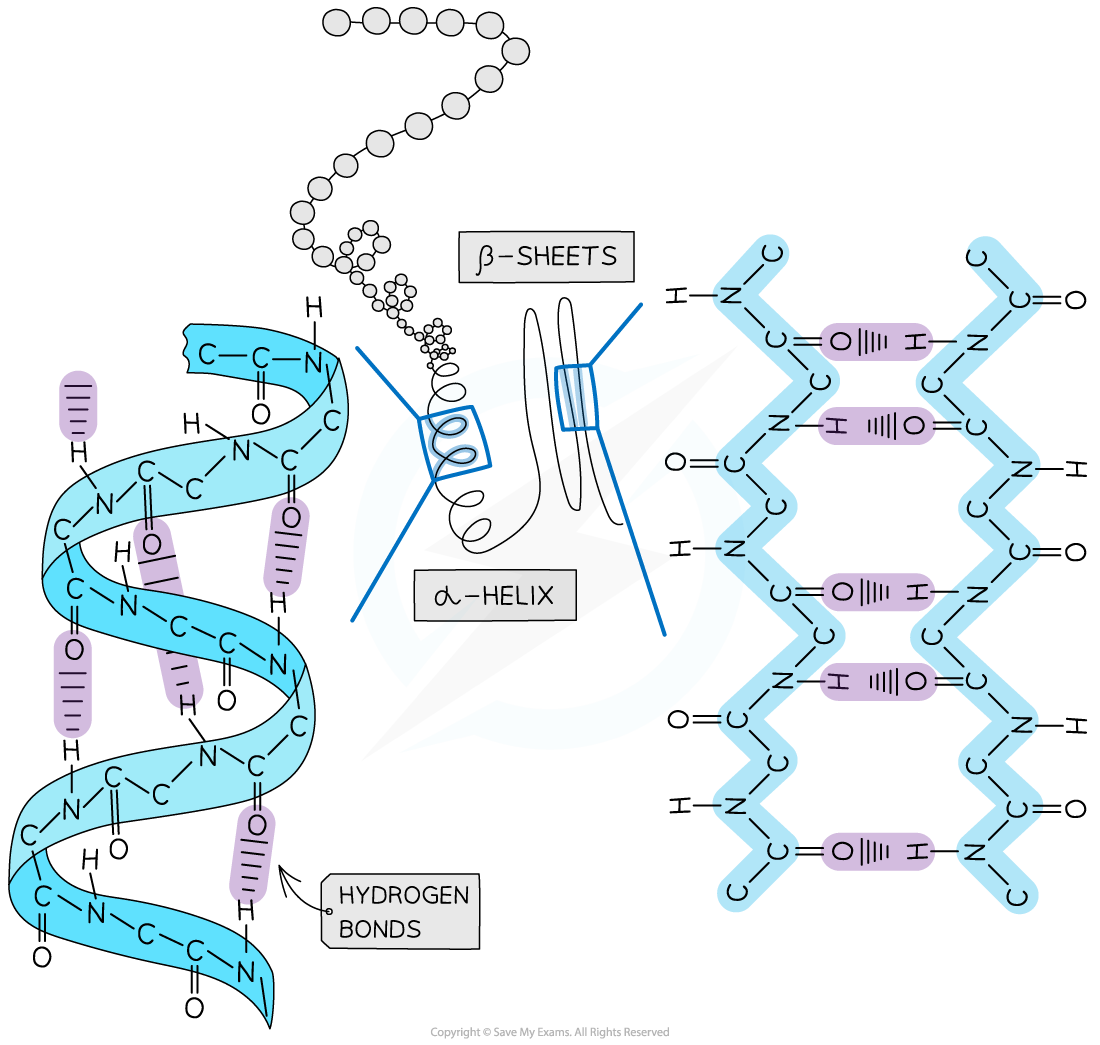
The secondary structure of a protein forms due to pleating and coiling of the amino acid chain
Secondary structure is held together by weak hydrogen bonds
Hydrogen bonds in the secondary structure form between carboxyl (C=O) groups and amino (N-H) groups
The pleating and coiling within a protein's secondary structure can give rise to:
alpha (α) helices (singular helix)
Beta (β)-pleated sheets
Tertiary Structure: Chemical Bonds
A protein's tertiary structure is the complex, three-dimensional shape into which the secondary structure folds
Tertiary structure gives proteins a very specific shape that is important for function, e.g. receptor sites on cell membranes and active sites in enzymes
Folding of the tertiary structure occurs due to interactions between R groups (side chains) of the amino acids, and interactions between R groups and the surrounding environment
Interactions that determine tertiary structure include:
hydrogen bonds between polar R-groups
Note that this differs from the location of the hydrogen bonds within the secondary structure of proteins
hydrophobic interactions occur between non-polar R-groups and water in the surrounding environment; this causes the amino acids that contain these R groups to position themselves on the inside of a protein
covalent bonds form between the R-groups of cysteine amino acids to form disulfide bridges
ionic bonds form between positively and negatively charged R-groups
amine and carboxyl groups within R-groups can become positively or negatively charged due to the binding or dissociation of hydrogen ions

Bonds | Level of structure | ||
|---|---|---|---|
Primary | Secondary | Tertiary | |
Peptide | ✓ | ✓ | ✓ |
Hydrogen |
| ✓ (between amino and carboxyl groups) | ✓ ( between R groups) |
Disulfide |
|
| ✓ |
Ionic |
|
| ✓ |
Hydrophobic interactions |
|
| ✓ |
Tertiary Structure: Amino Acids
Amino acids are either polar or non-polar, depending on the structure of their R-groups
Proteins with polar amino acids can form structures that are soluble in water
These proteins develop a globular structure with:
polar, hydrophilic amino acids arranged on the outside
non-polar, hydrophobic amino acids clustered in the centre
Proteins with polar amino acids are found in a variety of places within the cell, e.g.
on the surface of membranes where they must interact with water molecules in the environment
forming interior pores within the membrane, which creates hydrophilic channels for transport of polar molecules into and out of a cell
on the outside of enzymes, so that enzymes are soluble in aqueous environments
Non-polar amino acids can be found in regions of proteins that do not interact with aqueous solutions
E.g. integral proteins have regions with hydrophobic amino acids, helping them to embed in membranes
Examiner Tips and Tricks
Remember that the hydrogen bonds in tertiary structures are between the R groups, whereas in secondary structures the hydrogen bonds form between the amino and carboxyl groups.
Quaternary Structure
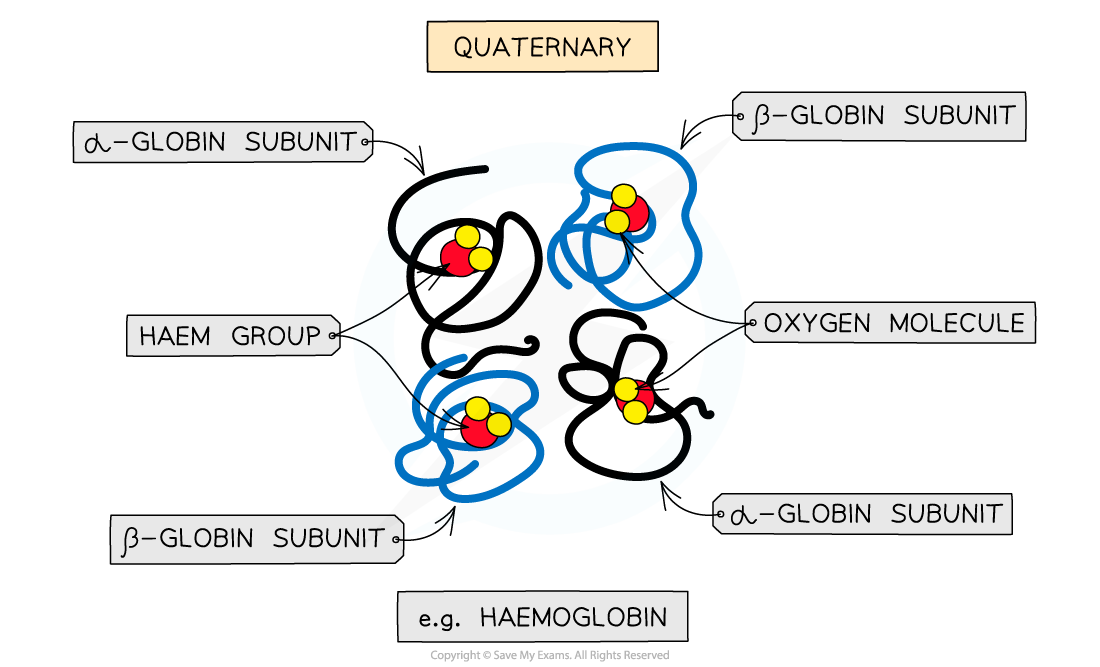
Some proteins have a quaternary structure, meaning that they contain multiple polypeptide chains functioning together as a single protein
Each polypeptide chain is referred to as a subunit
Proteins with only one polypeptide chain do not have a quaternary structure
The quaternary structure of a protein can be either conjugated or non-conjugated
Conjugated proteins contain non-protein components known as prosthetic groups, while non-conjugated proteins do not
Haemoglobin
Haemoglobin has a quaternary structure and is a conjugated protein
It has a quaternary structure, as it consists of four polypeptide subunits
It is conjugated because each subunit contains a prosthetic group
Each subunit has a prosthetic haem group which contains an iron ion (Fe2+)
Insulin and collagen
Insulin and collagen both have a quaternary structure and are non-conjugated proteins, meaning that they have no non-protein components
Insulin:
consists of two polypeptide subunits joined by disulfide bridges
Collagen:
is a fibrous protein consisting of three polypeptide subunits wound together in a helix shape
NOS: Technology allows imaging of structures that would be impossible to observe with the unaided senses. For example, cryogenic electron microscopy has allowed imaging of single-protein molecules and their interactions with other molecules
Cryogenic electron microscopy (cryo-EM) involves rapid freezing of protein solutions and then exposing them to many electrons to produce a microscopic image
The images can be used to recreate the 3D shape of proteins, allowing us to visualise how they interact with other molecules within a cellular environment
Cryo-EM has different applications depending on the type of protein or molecule being studied, so observations can be extremely purposeful and exact
Until recently proteins had to be crystallised to reconstruct and visualize them with X-ray crystallography; this posed problems such as:
crystallisation is time-consuming and can only work on single purified protein
some proteins do not crystallise
the structure has to be visualised outside of the cellular environment, which removes contextual information, e.g. interactions with other molecules

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
