Enzyme Reaction Rates: Skills (DP IB Biology) : Revision Note
Determining Enzyme Reaction Rates
Enzyme catalysed reactions can be affected by changes in pH, temperature or substrate concentration
The rate of reaction can be determined by measuring the rate of disappearance of a substrate or the rate of product accumulated in a given time period
This may be shown as a change in quantity (usually volume or mass) of substrate or product over a measured time period:

Or, if we cannot collect quantitative data on the amount of substrate or product, we can calculate the rate of reaction based on the time measured using the following equation:

1 ÷ time taken (seconds) and should include the units s⁻¹
A high rate of reaction is when the reaction happens in less time i.e. it is faster
A low rate of reaction is when the reaction happens in more time i.e. it is slower
The rate of a reaction is likely to change throughout a reaction as the substrate concentration will decrease as the reaction proceeds
This leads to a graph that starts out as a directly proportional straight line (the value on the X increases at the same rate as the value on the Y) but then plateaus as the reaction slows down
The steeper the line the faster the rate of reaction
The rate of reaction can be calculated from a graph plotted where the reaction time is shown on the X-axis and the quantity of product or substrate is shown on the Y-axis
Volume of a product produced against time graph
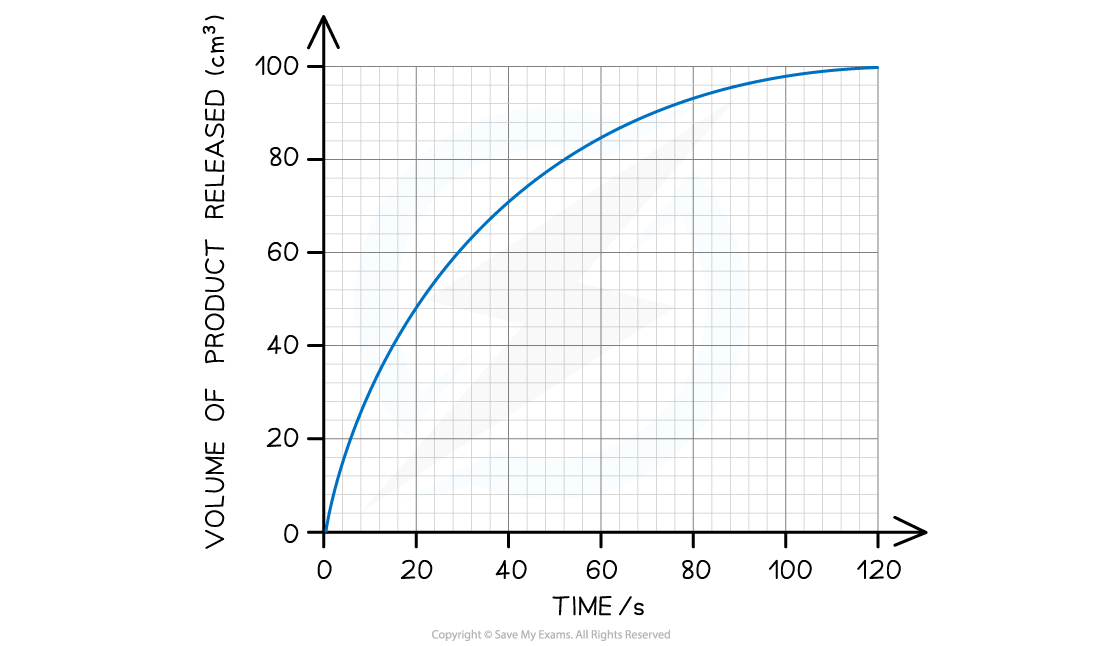
Graph produced when plotting the volume of a product produced against time
The gradient is calculated from a point on the graph and used as a measure of the rate of reaction at that point in time
A tangent must be drawn to calculate the change in x and y so the rate of reaction can be calculated
E.g. if calculating the initial rate of reaction
Place a ruler on the point of origin and draw a line that corresponds to the curve during the early part of the reaction
Extend the line as far as is convenient to perform the calculations e.g. to 60 seconds
Drawing a tangent to calculate initial rate of reaction diagram

Drawing a tangent against the line through the origin to calculate the initial rate of reaction
Calculating the rate of reaction
Once the tangent is drawn you can calculate the gradient of the line which is equal to the rate of the reaction
Initial rate = a ÷ b
Where
a = change in volume and
b = change in time
The units will be cm³ sec⁻¹ (this means volume per sec)
Calculating the rate of reaction from the tangent diagram

Rate of reaction is calculated by finding the gradient of the tangent from the origin

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
