Enzyme Action (DP IB Biology): Revision Note
Structure of Enzymes
The structure of enzymes
Enzyme catalysis involves molecular motion and the collision of substrates with the active site
For an enzyme-catalysed reaction to take place, substrates collide at random with the enzyme's active site
This must happen at the correct orientation and speed in order for a reaction to occur
Unsuccessful collisions can occur when the molecules are not correctly aligned with each other at the moment of collision
The molecules 'bounce' off each other and no reaction takes place
Some enzymes have two substrates that must each collide with a separate active site at the same time
Substrates bind to enzymes, forming a temporary enzyme-substrate complex
The active site of an enzyme has a specific shape and chemical properties to bind with a specific substrate
The reaction occurs within the enzyme-substrate complex which leads to changes in the chemical structure of the substrate
Products are formed, which detach and move away from the active site, which can be re-used
Enzyme action diagram

The active site of an enzyme has a specific shape to fit a specific substrate (when the substrate binds an enzyme-substrate complex is formed)
The specificity of an enzyme is a result of the complementary nature between the shape of the active site on the enzyme and its substrate(s)
The shape of the active site (and therefore the specificity of the enzyme) is determined by the complex 3D shape of the protein that makes up the enzyme
The active site is made of only a few amino acids but the interaction of these amino acids within the 3D shape of the enzyme ensures that catalysis can occur
This is achieved by:
Binding to the substrate molecule
Holding it in position for a chemical reaction to occur
Lowering the energy needed for the reaction to occur
Enzyme specificity diagram

An example of enzyme specificity – the enzyme catalase can bind to its substrate hydrogen peroxide as they are complementary in shape, whereas DNA polymerase is not
Formation of enzyme-substrate complex diagram

The temporary formation of an enzyme-substrate complex
Examiner Tips and Tricks
Don't forget that both enzymes and their substrates are highly specific to each other – this is known as enzyme-substrate specificity.
Induced-fit Binding
The induced-fit hypothesis
The original model explaining interactions between enzymes and their substrate molecules was called the lock-and-key model
This model proposed that the enzyme active site is precisely complementary to the shape of the substrate molecule
The substrate molecule therefore fits into the active site like a key in a lock
The modified model of enzyme activity is known as the ‘induced-fit hypothesis’
Although it is very similar to the lock and key hypothesis, in this model the enzyme and substrate interact with each other:
The enzyme and its active site (and sometimes the substrate) can change shape slightly as the substrate molecule enters the enzyme
These changes in shape are known as conformational changes
This ensures an ideal binding arrangement between the enzyme and substrate is achieved
This maximises the ability of the enzyme to catalyse the reaction
Induced-fit model diagram
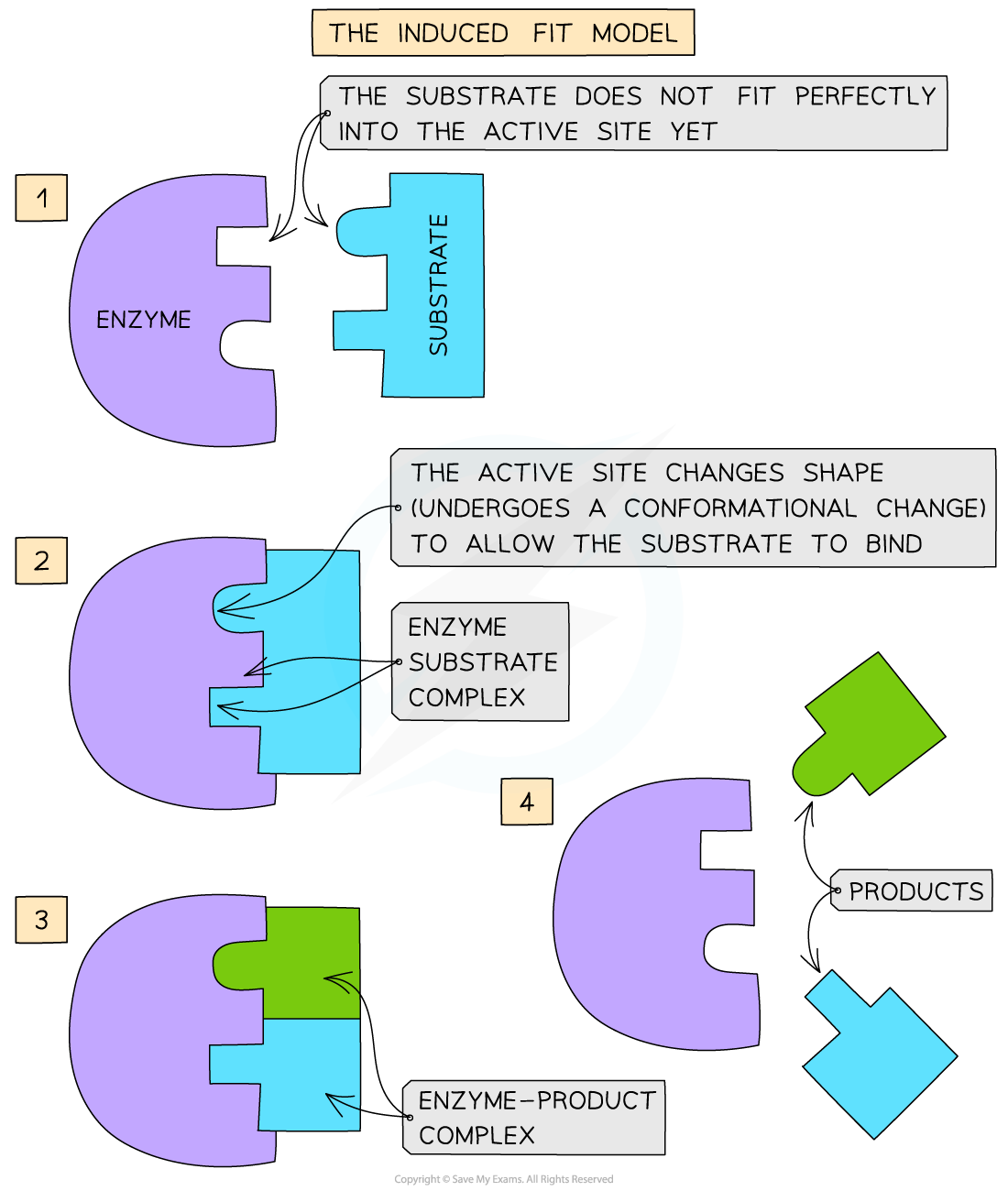
The induced-fit hypothesis of enzyme action
Examiner Tips and Tricks
Don't forget – our current understanding of enzyme-substrate interactions is based on the induced-fit hypothesis.
Enzyme Catalysis
The role of molecular motion and substrate-active site collisions
In order for the substrate molecule to collide with and ultimately bind to the enzyme active site, movement is required
This movement is the result of the kinetic energy that molecules have
The greater the kinetic energy of the molecules, the faster the movement and the higher the probability of enzyme and substrate colliding
This leads to more enzyme-substrate complexes forming and the production of more product molecules
In some cases, large substrate molecules are immobilised, while in other cases it is possible to immobilise enzymes by embedding them in membranes
These immobilised enzymes can be used in a range of industries such as food processing, environmental management, pharmaceuticals and manufacturing processes
There are different methods by which enzymes can be immobilised including:
Attachment to an inert substance e.g. glass
Entrapment within a matrix e.g. alginate gel
Entrapment within a partially permeable membrane
Examples of immobilised enzymes diagram


There are many different ways in which enzymes can be immobilised
Advantages of immobilised enzymes
There is no enzyme in the product (the product is uncontaminated) and therefore there is no need to further process or filter the end product
The immobilised enzyme can be reused multiple times which is both efficient and cost-effective (many enzymes are expensive)
Reusing the enzyme also avoids the need to separate the enzyme from the product in downstream processing
Immobilised enzymes have a greater tolerance of temperature and pH changes (immobilisation often makes enzymes more stable)
Substrates can be exposed to higher enzyme concentrations than when using enzymes in solution, increasing the rate of throughput
Conditions can be controlled carefully, allowing immobilised enzymes to function close to their optimum conditions and be more stable
Denaturation: Enzymes
Enzymes can be denatured when it is exposed to high temperatures or extremes of pH
Bonds (e.g. hydrogen bonds) holding the enzyme molecule in its precise 3D shape start to break
Take note that the peptide bonds holding the amino acids together are not broken
This causes the 3-dimensional shape of the protein (i.e. the enzyme) to change
This permanently changes the shape of the active site, preventing the substrate from binding
Denaturation has occurred if the substrate can no longer bind
The reaction that was previously catalysed now no longer takes place
Denaturation often causes the enzyme to become insoluble and form a precipitate
Very few human enzymes can function at temperatures above 50°C
This is because humans maintain a body temperature of about 37°C, therefore even temperatures exceeding 40°C will cause the denaturation of enzymes
High temperatures cause increased vibrations in the bonds between the R-groups of amino acids so they start to break, changing the conformation of the enzyme
Examiner Tips and Tricks
Don't forget that enzymes are always proteins and so anything that could denature a protein, rendering it non-operational (extremes of heat, temperature, pH etc.) would also denature an enzyme. Avoid using the term 'destroyed' or saying that the enzyme is 'killed' when describing the disruption to enzyme structure.

Unlock more, it's free!
Did this page help you?