Globular & Fibrous Proteins (DP IB Biology) : Revision Note
Globular & Fibrous Proteins
Globular
Globular proteins are compact, roughly spherical (circular) in shape and soluble in water
Globular proteins form a spherical shape when folding into their tertiary structure because:
Their non-polar hydrophobic R-groups are orientated towards the centre of the protein away from the aqueous surroundings and
Their polar hydrophilic R-groups orientate themselves on the outside of the protein
This orientation enables globular proteins to be (generally) soluble in water as the water molecules can surround the polar hydrophilic R-groups
The solubility of globular proteins in water means they play important physiological roles as they can be easily transported around organisms and be involved in metabolic reactions
The folding of the protein due to the interactions between the R-groups results in globular proteins having specific shapes. This also enables globular proteins to play physiological roles, for example, enzymes can catalyse specific reactions and immunoglobulins can respond to specific antigens
Some globular proteins are conjugated proteins that contain a prosthetic group eg. haemoglobin which contains the prosthetic group called haem
Insulin
The first protein to have its sequence determined by scientists was the hormone insulin
Insulin is a globular protein produced in the pancreas. It plays an important role in the control of blood glucose concentration
It consists of two polypeptide chains
Polypeptide A has 21 amino acid residues
Polypeptide B has 30 amino acid residues
The two polypeptide chains are held together by three disulfide bridges
Fibrous
Fibrous proteins are long strands of polypeptide chains that have cross-linkages due to hydrogen bonds
They have little or no tertiary structure
Due to the large number of hydrophobic R-groups fibrous proteins are insoluble in water
Fibrous proteins have a limited number of amino acids with the sequence usually being highly repetitive
The highly repetitive sequence creates very organised structures that are strong and this along with their insolubility property, makes fibrous proteins very suitable for structural roles, for example, keratin that makes up hair, nails, horns and feathers and collagen which is a connective tissue found in skin, tendons and ligaments
Collagen
Collagen is the most common structural protein found in vertebrates
It has a flexible structure, forming connective tissues:
Tendons
Cartilage
Ligaments
Bones
Teeth
Skin
Walls of blood vessels
Cornea of the eye
Collagen is an insoluble fibrous protein
Collagen is formed from three polypeptide chains closely held together by hydrogen bonds to form a triple helix; the hydrogen bonds give great tensile strength
Each polypeptide chain is a helix shape and contains about 1000 amino acids with glycine, proline and hydroxyproline being the most common
Along with hydrogen bonds forming between the three chains there are also covalent bonds present
Covalent bonds also form cross-links between R-groups of amino acids in interacting triple helices when they are arranged parallel to each other. The cross-links hold the collagen molecules together to form fibrils
The collagen molecules are positioned in the fibrils so that there are staggered ends which provide strength
When many fibrils are arranged together they form collagen fibres
Collagen fibres are positioned so that they are lined up with the forces they are withstanding
Globular and fibrous proteins diagram
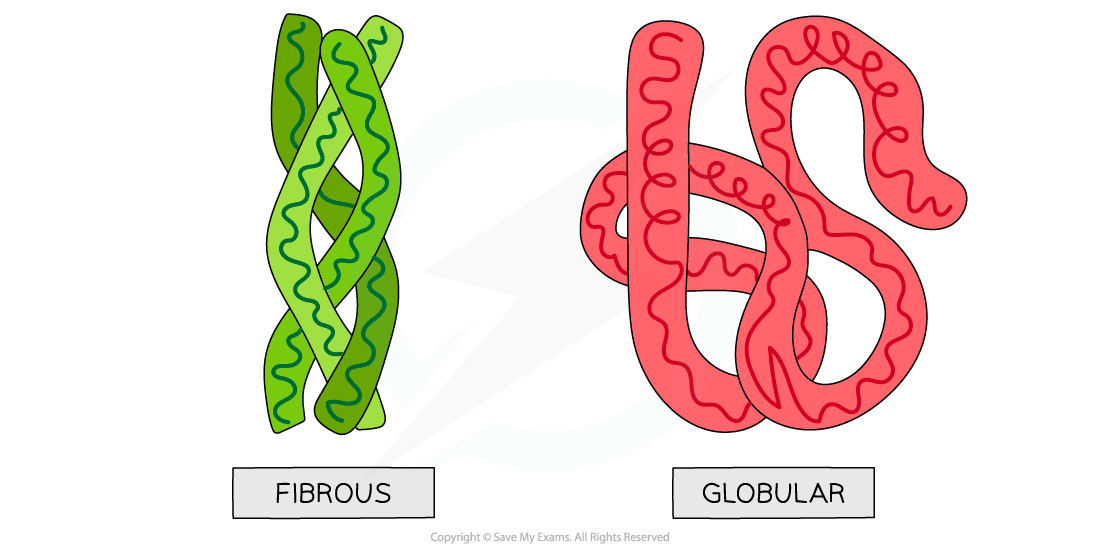
Globular and fibrous protein models illustrating the roughly spherical shape of globular proteins and the long, stranded shape of fibrous proteins
Comparison of globular & fibrous tertiary proteins table

Examiner Tips and Tricks
To distinguish between the two proteins, learn SAFES (Shape, Amino acid sequence, Function, Examples and Solubility).

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
