Osmosis: Skills (DP IB Biology) : Revision Note
Changes in Plant Tissue due to Water Movement
Experimental design; accurate quantitative measurements in osmosis experiments are essential
Planning is an essential part of experimental biology, it will help ensure that valid conclusions can be made
Preliminary (meaning "to come before") research must be completed to ensure the experiment design considers:
The results that will be collected
Quantitative data allows more valid conclusions to be made
Qualitative data (descriptive) can be useful to support the conclusions
How measurements will be made so they are as precise and as accurate as possible
The choice of apparatus and techniques should be based on the science surrounding the issue being investigated
How many repeats will be undertaken to ensure the data collected is reliable
The variables that will be tested and need to be controlled
Once the preliminary research has been completed then preliminary studies can be conducted to further aid the experimental design
These studies are very important for:
Identifying additional variables that affect the experiment
Finding the best way to control these variables
Deciding on the quantities and volumes of substances that are needed so that you do not run out of reactants/reagents
Any experiment conducted without preliminary research or studies is likely to be invalid as the other variables that affect the results in the experiment will not have been identified and controlled
Estimation of osmotic concentration in tissues by bathing samples in hypotonic and hypertonic solutions
The osmotic concentration (or solute concentration) in tissues can be estimated by bathing samples of plant tissue in solutions of different tonicity
A hypotonic solution has a lower osmotic concentration than the tissue being bathed in it (so the tissue will increase in mass or length) whereas a hypertonic solution has a higher osmotic concentration (so the tissue will decrease in mass or length)
An isotonic solution will have the same osmotic concentration as the tissue (so the mass or length will remain unchanged)
It is possible to investigate the effects of immersing plant tissue in solutions of different osmotic concentrations and to use the results to estimate the osmotic concentration of the plant tissue itself
The most common osmosis practical of this kind involves cutting cylinders of potato and placing them into solutions with a range of different osmotic concentrations
Usually sucrose solutions of increasing concentration – at least 5 different concentrations are usually required
Apparatus
Potato x 2 (same variety)
Cork borer (e.g. 5mm)
White tile
Scalpel
10cm ruler or vernier calipers
Weighing balance (2dp)
10 cm³ sucrose solution (0 mol/dm³, 0.25 mol/dm³, 0.5 mol/dm³, 0.75 mol/dm³, 1.00 mol/dm³)
5 test tubes (in test tube rack)
10 cm³ measuring cylinder
Paper towels
Method
The required number of potato cylinders are cut
At least 5 for each of the solutions you are testing to ensure you have sufficient repeats
They are all cut to the same length and, once blotted dry to remove any excess moisture, their initial mass is measured and recorded before placing into the solutions
The potato cylinders are left in the solutions for a set amount of time (e.g. 30 minutes), usually in a water bath (set at around 30o)
The solutions are prepared by serial dilutions of a specific solute concentration determined during the preliminary research/trials)
The cylinders are then removed and dried
This is done to remove excess liquid
The final length and mass of each potato cylinder is then measured and recorded



You will need to use apparatus appropriately to measure out the volumes of your solutions and record your measurements
Analysis
The percentage change in mass for each potato cylinder is calculated and then plotted

To find the percentage change in mass, the change in mass must be divided by the initial mass and then multiplied by 100

A positive percentage change in mass indicates that the potato has gained water by osmosis
A positive percentage change in mass indicates that the potato has gained water by osmosis (net movement of water from the solution into the potato) meaning the solution had a lower osmotic concentration than the potato
The gain of water makes the potato cells turgid, as the water exerts turgor pressure (or hydrostatic pressure) on the cell walls – the potatoes will feel hard
A negative percentage change suggests the opposite, that is, the solution had a higher osmotic concentration than the potato
The potato cylinder in the strongest sucrose concentration will have decreased in mass the most as there is the greatest concentration gradient in this tube between the potato cells (lower osmotic concentration) and the sucrose solution (higher osmotic concentration)
More water molecules will move out of the potato cells by osmosis, making them flaccid and decreasing the mass of the potato cylinder – the potato cylinders will feel floppy
If looked at underneath the microscope, cells from this potato cylinder might be plasmolysed, meaning the cell membrane has pulled away from the cell wall
If there is a potato cylinder that has neither increased nor decreased in mass, it means there was no overall net movement of water into or out of the potato cells
The solution that this particular potato cylinder was in had the same osmotic concentration as the solution found in the cytoplasm of the potato cells, so there was no concentration gradient and therefore no net movement of water into or out of the potato cells
The concentration of sucrose inside the potato cylinders can be found if a graph is drawn showing how the percentage change in mass changes with the concentration of sucrose solution
The point at which the line of best fit crosses the x-axis is the concentration of sucrose inside the potato cylinders
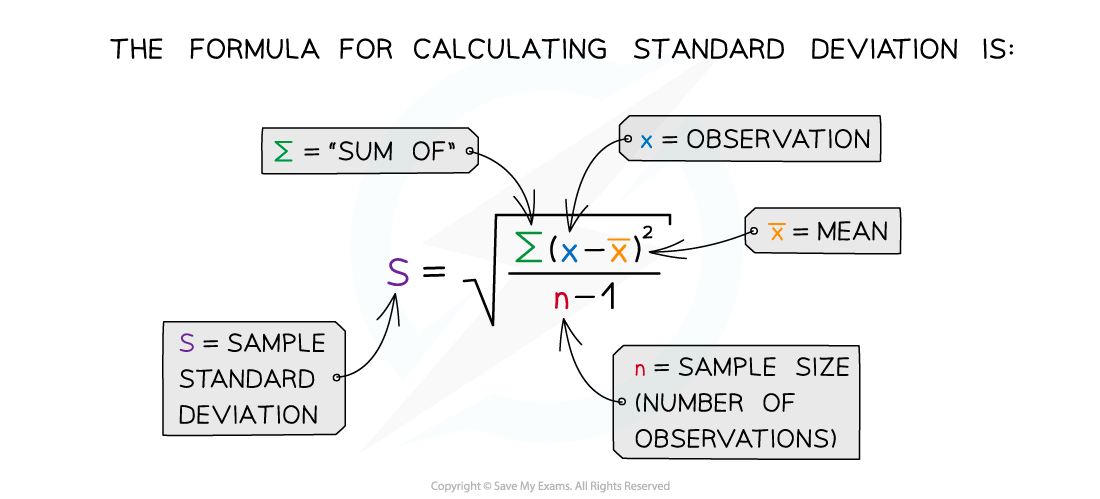
Calculating the standard deviation and standard error for the results of this experiment would allow the reliability of the length and mass measurements to be compared
Standard deviation
It is important to have sufficient repeats when conducting experiments, like the one above, in order to ensure reliable results
These repeat values can be used to calculate a mean mass for the potato cylinders in each sucrose concentration
The mean is a more informative statistic when it is provided alongside standard deviation
Standard deviation measures the spread of data around the mean value
This is very useful when comparing consistency between different data sets during data analysis
The standard deviation can be calculated using the following formula:
Standard error
The standard error gives an indication of how close the sample mean is to the true population mean
A large sample size results in a smaller standard error and the closer the sample mean will be to the true population mean
Standard error (SE) can be calculated by dividing the standard deviation (S) by the square root of the sample size (n):
When graphs of mean values are drawn, the standard error can be shown as error bars added to each plotted value
This demonstrate the deviation of the sample mean from the true population mean
Error bars will extend above and below the data points to indicate variability
If error bars overlap then it suggest that the difference between the mean values is not significant while non-overlapping error bars indicate a significant difference between the means
Graph to show the use of error bars in an osmosis investigation

In the graph above, there is no overlap between the error bars for the plotted values of sucrose concentrations between 0 and 0.6 mol dm-3, indicating a significant difference between these means
The error bars for a sucrose concentration between 0.7 and 1.0 mol dm-3 do overlap, indicating no significant difference between the means
Examiner Tips and Tricks
Note that you are not required to memorise the formulae for calculating these statistics. You do however, need to know how to use these statistical values to help analyse experimental data.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
