Rutherford Scattering
- Evidence for the structure of the atom was discovered by Ernest Rutherford in the beginning of the 20th century from the study of α-particle scattering
- The experimental setup consists of alpha particles fired at thin gold foil and a detector on the other side to detect how many particles deflected at different angles

α-particle scattering experiment set up
- α-particles are the nucleus of a helium atom and are positively charged

When α-particles are fired at thin gold foil, most of them go straight through but a small number bounce straight back
- From this experiment, Rutherford results were:
- The majority of α-particles went straight through (A)
- This suggested the atom is mainly empty space
- Some α-particles deflected through small angles of < 10o
- This suggested there is a positive nucleus at the centre (since two positive charges would repel)
- Only a small number of α-particles deflected straight back at angles of > 90o (C)
- This suggested the nucleus is extremely small and this is where the mass and charge of the atom is concentrated
- It was therefore concluded that atoms consist of small dense positively charged nuclei, surrounded by negatively charged electrons

An atom: a small positive nucleus, surrounded by negative electrons
- (Note: The atom is around 100,000 times larger than the nucleus!)
Worked example
In an α-particle scattering experiment, a student set up the apparatus below to determine the number n of α-particle incident per unit time on a detector held at various angles θ. Which of the following graphs best represents the variation of n with θ from 0 to 90°?
Which of the following graphs best represents the variation of n with θ from 0 to 90°?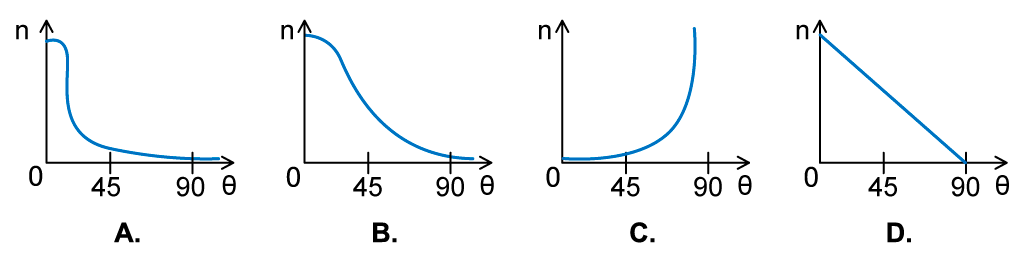
ANSWER: A
- The Rutherford scattering experience directed parallel beams of α-particles at gold foil
- The observations were:
- Most of the α-particles went straight through the foil
- The largest value of n will therefore be at small angles
- Some of the α-particles were deflected through small angles
- n drops quickly with increasing angle of deflection θ
- These observations fit with graph A






