Energy Levels & Photon Emission (AQA AS Physics): Revision Note
Exam code: 7407
Line Spectra & Energy Levels
Energy levels can be represented as a series of horizontal lines
The line at the bottom with the greatest negative energy represents the ground state
The lines above the ground state with decreasing energies represent excited states
The line at the top, usually 0 V or infinity
, represents the ionisation energy
Energy Levels in a Hydrogen Atom
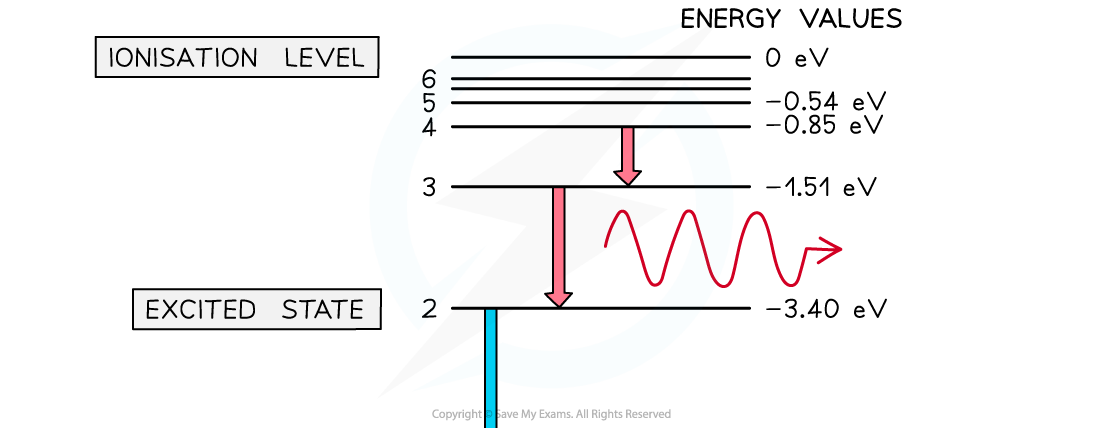

A photon is emitted when an electron moves from a higher energy state to a lower energy state. The energy of the emitted photon is equal to the difference in energy between the energy levels in the transition.
Line Spectra
Line spectra occur when excited atoms emit light of certain wavelengths which correspond to different colours
The emitted light can be observed as a series of lines with spaces in between
These series of lines are called line or atomic spectra
Each element produces a unique set of spectral lines
No two elements emit the same set of spectral lines, therefore, elements can be identified by their line spectrum
There are two types of line spectra: emission spectra and absorption spectra
Emission Spectra
When an electron transitions from a higher energy level to a lower energy level, this results in the emission of a photon
Each transition corresponds to a different wavelength of light and this corresponds to a line in the spectrum
The resulting emission spectrum contains a set of discrete wavelengths, represented by coloured lines on a black background
Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation:

Where:
ΔE = change in energy level (J)
h = Planck’s constant (J s)
f = frequency of photon (Hz)
c = the speed of light (m s-1)
λ = wavelength of the photon (m)
Line spectra provide evidence that electrons in atoms can only transition between discrete energy levels

Emission spectrum of Hydrogen gas
Absorption Spectra
An atom can be raised to an excited state by the absorption of a photon
When white light passes through a cool, low pressure gas it is found that light of certain wavelengths are missing
This type of spectrum is called an absorption spectrum
An absorption spectrum consists of a continuous spectrum containing all the colours with dark lines at certain wavelengths
These dark lines correspond exactly to the differences in energy levels in an atom
When these electrons return to lower levels, the photons are emitted in all directions, rather than in the original direction of the white light
Therefore, some wavelengths appear to be missing
The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element
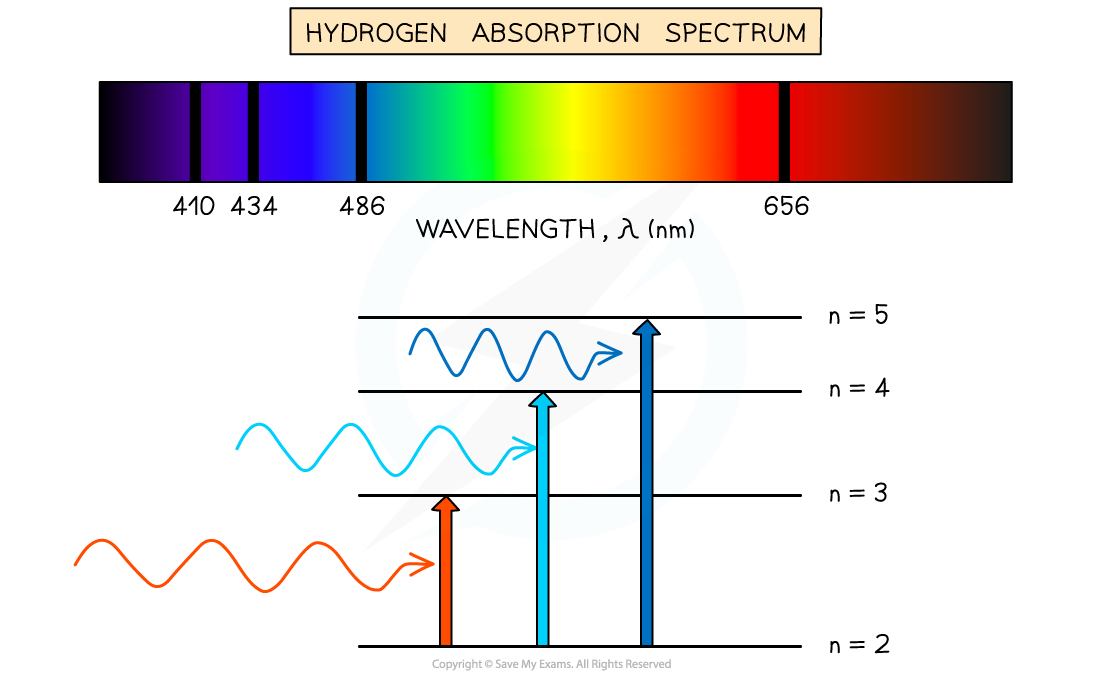
Absorption spectrum of Hydrogen gas
Difference in Discrete Energy Levels
The difference between two energy levels is equal to a specific photon energy
The energy (hf) of the photon is given by:
ΔE = hf = E2 - E1
Where:
E1 = Energy of the higher level (J)
E2 = Energy of the lower level (J)
h = Planck’s constant (J s)
f = Frequency of photon (Hz)
Using the wave equation, the wavelength of the emitted, or absorbed, radiation can be related to the energy difference

This equation shows that the larger the difference in energy between two levels ΔE (E2 - E1) the shorter the wavelength λ and vice versa
Worked Example
Some electron energy levels in atomic hydrogen are shown below.

The longest wavelength produced as a result of electron transitions between two of the energy levels is 4.0 × 10–6 m.
a) Draw and mark:
The transition giving rise to the wavelength of 4.0 × 10–6 m with letter L.
The transition giving rise to the shortest wavelength with letter S.
b) Calculate the wavelength for the transition giving rise to the shortest wavelength.
Answer:
Part (a)
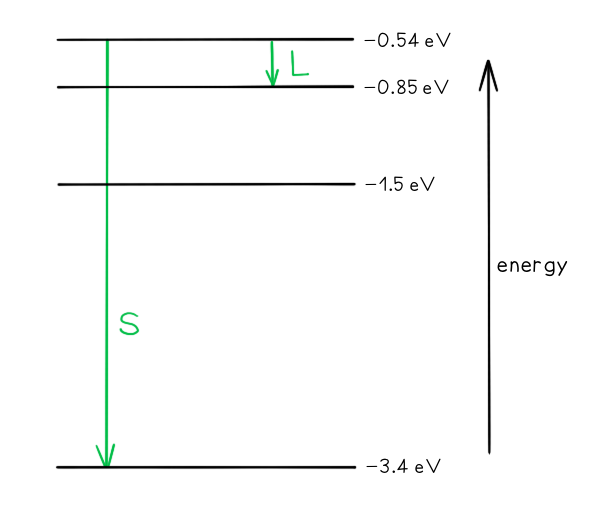
Photon energy and wavelength are inversely proportional
Therefore, the largest energy change corresponds to the shortest wavelength (line S)
The smallest energy change corresponds to the longest wavelength (line L)
Part (b)

Examiner Tips and Tricks
It doesn't matter which energy level you assign as E1 or E2, you just need to know the difference between the energy of the two energy levels.

Unlock more, it's free!
Did this page help you?