Calculating Uncertainties (AQA AS Physics) : Revision Note
Uncertainty
There is always a degree of uncertainty when measurements are taken; the uncertainty can be thought of as the difference between the actual reading taken (caused by the equipment or techniques used) and the true value
Uncertainties are not the same as errors
Errors can be thought of as issues with equipment or methodology that cause a reading to be different from the true value
The uncertainty is a range of values around a measurement within which the true value is expected to lie, and is an estimate
For example, if the true value of the mass of a box is 950 g, but a systematic error with a balance gives an actual reading of 952 g, the uncertainty is ±2 g
These uncertainties can be represented in a number of ways:
Absolute Uncertainty: where uncertainty is given as a fixed quantity
Fractional Uncertainty: where uncertainty is given as a fraction of the measurement
Percentage Uncertainty: where uncertainty is given as a percentage of the measurement
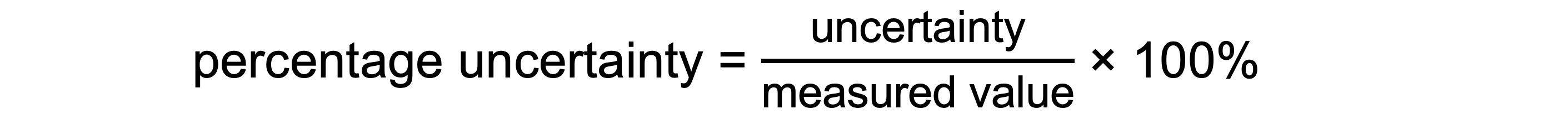
To find uncertainties in different situations:
The uncertainty in a reading: ± half the smallest division
The uncertainty in a measurement: at least ±1 smallest division
The uncertainty in repeated data: half the range i.e. ± ½ (largest - smallest value)
The uncertainty in digital readings: ± the last significant digit unless otherwise quoted
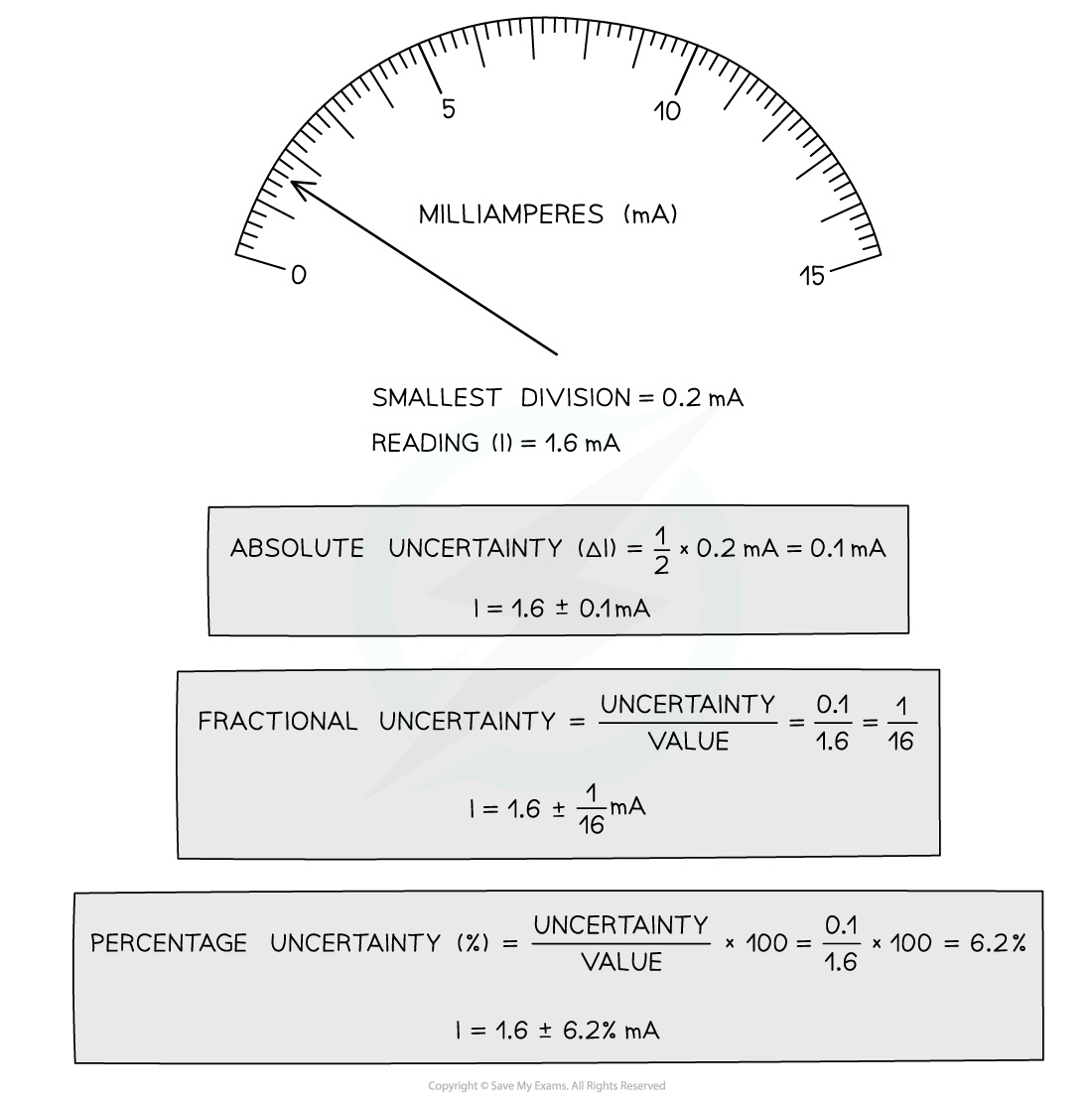
How to calculate absolute, fractional and percentage uncertainty
Always make sure your absolute or percentage uncertainty is to the same number of significant figures as the reading
Combining Uncertainties
When combining uncertainties, the rules are as follows:
Adding / Subtracting Data
Add together the absolute uncertainties
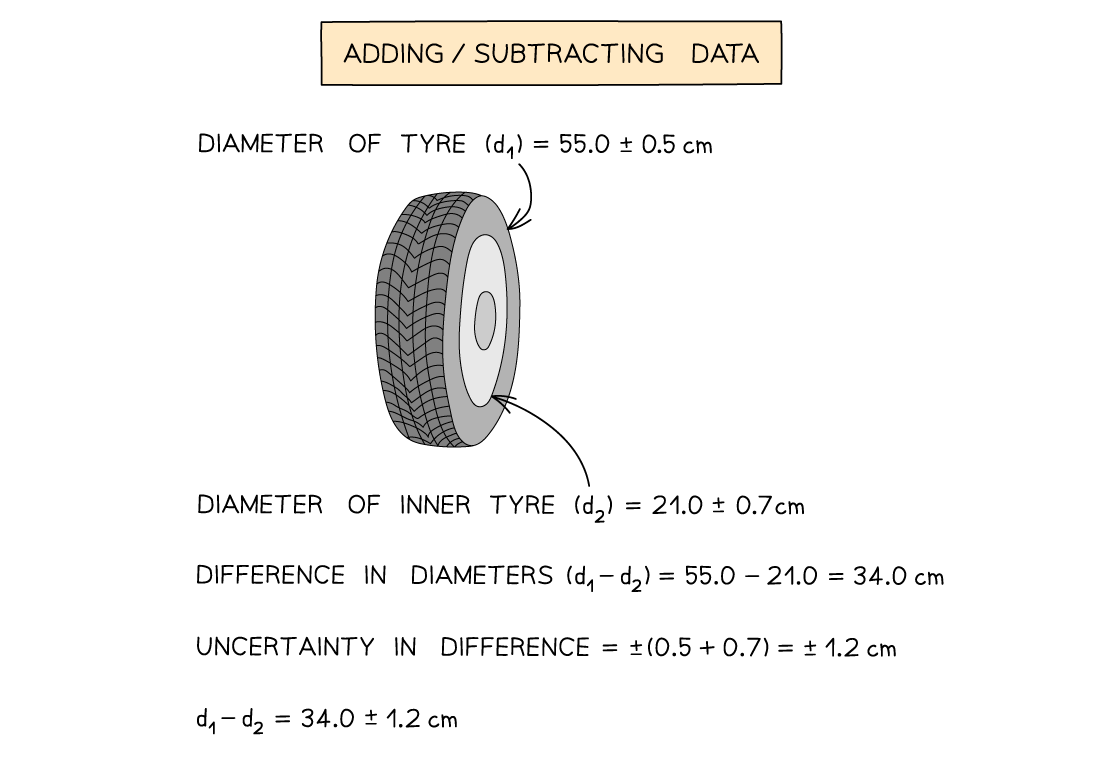
Multiplying / Dividing Data
Add the percentage or fractional uncertainties

Raising to a Power
Multiply the percentage uncertainty by the power

Worked Example
A student achieves the following results in their experiment for the angular frequency, ω.
0.154, 0.153, 0.159, 0.147, 0.152
Calculate the percentage uncertainty in the mean value of ω.
Answer:
Step 1: Calculate the mean value
mean ω = = 0.153 rad s–1
Step 2: Calculate half the range (this is the uncertainty for multiple readings)
× (0.159 – 0.147) = 0.006 rad s–1
Step 3: Calculate percentage uncertainty
× 100 % =
× 100 %
× 100 % = 3.92 %
Examiner Tips and Tricks
Remember:
Absolute uncertainties (denoted by Δ) have the same units as the quantity
Percentage uncertainties have no units
The uncertainty in numbers and constants, such as π, is taken to be zero
Uncertainties in trigonometric and logarithmic functions will not be tested in the exam, so just remember these rules and you’ll be fine!

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

