Ozone Depletion (Cambridge (CIE) AS Environmental Management): Revision Note
Exam code: 8291
Ozone Depletion
Ozone is a molecule composed of three oxygen atoms (O₃):
It is primarily found in the Earth's stratosphere, a layer of the atmosphere located approximately 10 to 50 kilometres above the Earth's surface
Ozone plays a crucial role in protecting life on Earth by absorbing a significant portion of the Sun's harmful UV radiation
How Ozone Depletion Occurs
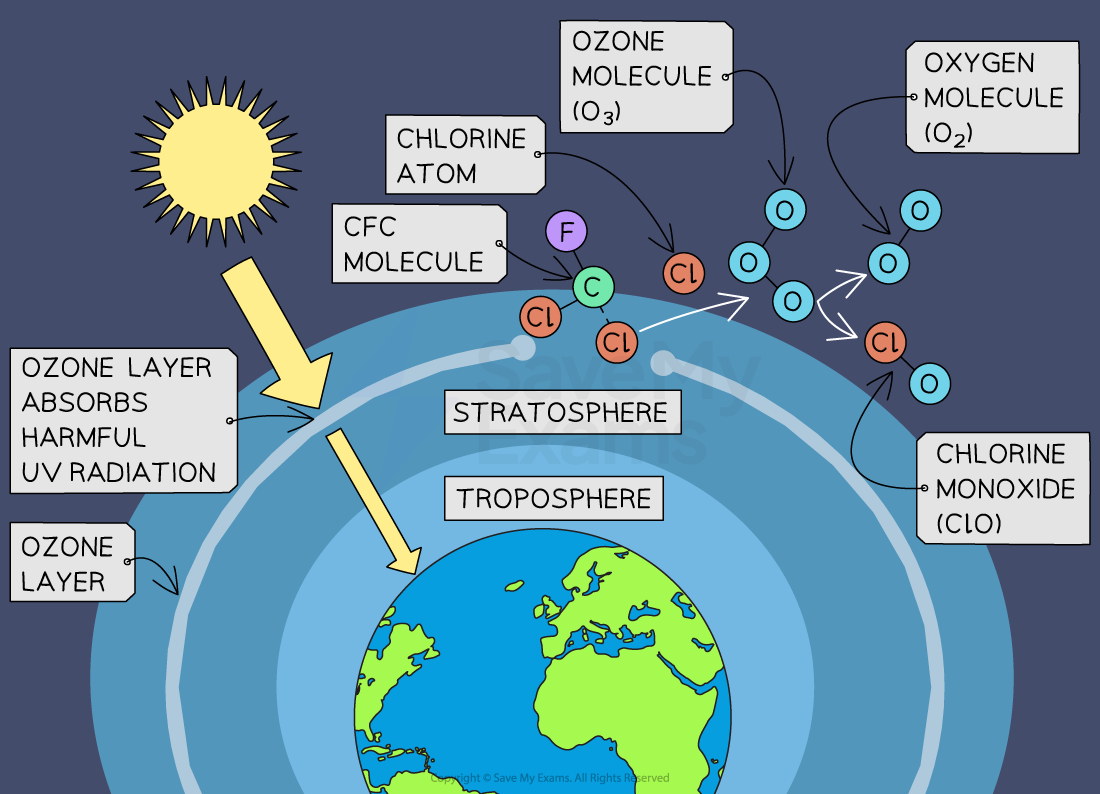
Ozone depletion refers to the gradual thinning of the ozone layer in the Earth's stratosphere, primarily due to human-made chemicals known as chlorofluorocarbons (CFCs):
Ozone depletion occurs through a series of chemical reactions involving CFCs and UV radiation in the stratosphere
This process leads to the destruction of ozone molecules, resulting in the formation of an ozone hole, particularly over polar regions

How the ozone depletion process occurs:
Chlorofluorocarbons (CFCs) from aerosols, refrigerants and industrial processes are stable compounds and do not readily degrade in the lower atmosphere (troposphere)
As CFCs are released into the atmosphere, they gradually drift upward into the stratosphere
In the stratosphere, CFC molecules are broken down by ultraviolet (UV) radiation from the sun, releasing chlorine atoms
Chlorine atoms are highly reactive and can catalytically destroy ozone molecules
A single chlorine atom can destroy thousands of ozone molecules before being removed from the stratosphere
This process initiates a chain reaction where ozone (O₃) is converted into oxygen (O₂), leading to a reduction in the concentration of ozone molecules in the stratosphere
Chlorine atoms released from CFCs can persist in the stratosphere for many years, continuing to catalyse ozone depletion
Measurement of Ozone Concentration
Monitoring ozone concentration is crucial for understanding the extent of ozone depletion and its impacts on the environment and human health
Ozone concentration is commonly measured using the Dobson Unit (DU), named after G.M.B. Dobson who developed the first ozone spectrophotometer:
The Dobson Unit (DU) provides a standardised measure of the ozone layer's thickness
The Dobson Unit represents the thickness of the ozone layer if it were compressed into a layer of pure ozone at standard temperature and pressure
Ozone monitoring stations around the world use Dobson spectrophotometers or similar instruments to measure the total ozone column above a specific location
Ozone Holes
An ozone hole represents an area of significantly reduced ozone concentration in the stratosphere, usually observed over polar regions
Ozone holes are characterised by ozone levels falling below a certain threshold, often measured in Dobson Units, indicating severe ozone depletion in these regions:
In these regions the average concentration of ozone is significantly reduced, typically below 100 Dobson Units
Ozone holes are most commonly observed over the polar regions, particularly over Antarctica during the Southern Hemisphere's spring (September to November)
Factors Contributing to Ozone Depletion Over Antarctica
Ozone depletion is particularly pronounced over Antarctica, leading to the formation of an "ozone hole" during the Southern Hemisphere's spring
Several factors, including temperature conditions, the presence of specific cloud formations and atmospheric circulation patterns contribute to the significant ozone depletion observed over Antarctica:
Temperature: Extremely low temperatures in the Antarctic stratosphere during winter create conditions that favour the formation of polar stratospheric clouds (PSCs)
Polar Stratospheric Clouds (PSCs): These clouds provide a surface for chemical reactions that convert stable gas molecules containing chlorine (e.g. hydrogen chloride and chlorine nitrate) into reactive forms (e.g. free chlorine atoms), which accelerate the destruction of ozone molecules
The presence of PSCs amplifies ozone depletion within the Antarctic polar vortex
Polar Vortex: The polar vortex is a persistent, large-scale circulation pattern that forms over the polar regions during winter:
This vortex isolates the air within it, preventing exchange with air from lower latitudes and enhancing the concentration of ozone-depleting substances within the vortex

Examiner Tips and Tricks
Remember - an "ozone hole" is not a literal hole in the atmosphere. It is an area where ozone is much less concentrated i.e. the concentration of ozone has fallen below a certain level. This means harmful UV radiation can reach the Earth's surface more easily in these areas, as there is less protective ozone to block these UV rays.

Unlock more, it's free!
Did this page help you?