Structure & Composition of the Atmosphere (Cambridge (CIE) AS Environmental Management): Revision Note
Exam code: 8291
Major Components of Earth’s Atmosphere
The atmosphere is primarily composed of nitrogen (about 78%) and oxygen (about 21%)
These two gases make up the majority of the atmosphere and play vital roles in supporting life on Earth
In addition to nitrogen and oxygen, the atmosphere contains smaller amounts of other gases, including carbon dioxide, argon, water vapour, and various trace gases
Carbon dioxide, although present in relatively low concentrations (around 0.04%), is essential for maintaining the greenhouse effect, which helps regulate the Earth's temperature, and is essential for photosynthesis
Argon is an inert gas that does not participate in chemical reactions but contributes to the overall composition of the atmosphere (around 0.96%)
Water vapour is a variable component that plays a crucial role in the Earth's weather patterns, the formation of clouds and precipitation, and photosynthesis
Trace gases, such as methane, ozone, and nitrous oxide, are present in even smaller quantities but can have significant impacts on climate and atmospheric chemistry

Structure of Earth’s Atmosphere
The atmosphere is stratified into different layers based on temperature changes
The inner layers of the atmosphere, where most interactions related to living systems occur, are the troposphere and the stratosphere
The troposphere is the lowest layer of the atmosphere, extending from the Earth's surface up to about 10 kilometres
It is where weather phenomena, such as cloud formation, precipitation, and the mixing of gases, mainly occur
The troposphere contains the highest concentration of water vapour, carbon dioxide, and other trace gases that are important for the functioning of living systems and the regulation of climate
The air in the troposphere is heated from the ground by longwave radiation so it is warmest near the Earth’s surface and cools with increasing altitude
Above the troposphere is the stratosphere, which extends from approximately 10 kilometres to 50 kilometres above the Earth's surface
The stratosphere contains the ozone layer, a region with a higher concentration of ozone molecules that absorb and block most of the Sun's harmful ultraviolet (UV) radiation (a form of shortwave radiation)
This layer is crucial for protecting life on Earth from excessive UV radiation and has important implications for the health of ecosystems
The mesosphere is the zone in which most meteors burn up as a result of colliding with gas molecules
In this layer, temperature decreases as altitude increases
In the thermosphere, temperature increases with altitude
It is the reactions occurring in the inner layers of the atmosphere, particularly the troposphere and the stratosphere, are crucial for maintaining the balance of gases, regulating climate patterns, and supporting life
Within the troposphere, chemical reactions involving pollutants, greenhouse gases, and atmospheric particles can impact air quality and climate
In the stratosphere, chemical reactions involving ozone play a vital role in maintaining the ozone layer and protecting the Earth from harmful UV radiation

The Ozone Layer
Ozone is a molecule composed of three oxygen atoms (O₃)
It is primarily found in the Earth's stratosphere, a layer of the atmosphere located approximately 10 to 50 kilometres above the Earth's surface
Ozone plays a crucial role in protecting life on Earth by absorbing a significant portion of the Sun's harmful UV radiation
Dangers of UV Radiation
Exposure to high levels of UV radiation can be dangerous for life in the following ways:
Excessive UV radiation damages photosynthetic organisms, such as phytoplankton, by causing DNA damage and inhibiting photosynthesis
This can lead to a decrease in primary productivity, particularly in aquatic ecosystems
Excessive UV radiation can also lead to various health issues in humans and other animals by damaging cells and tissues - these issues can include:
Cataracts
Skin cancer
Sunburn
Premature skin ageing
Damage to immune systems
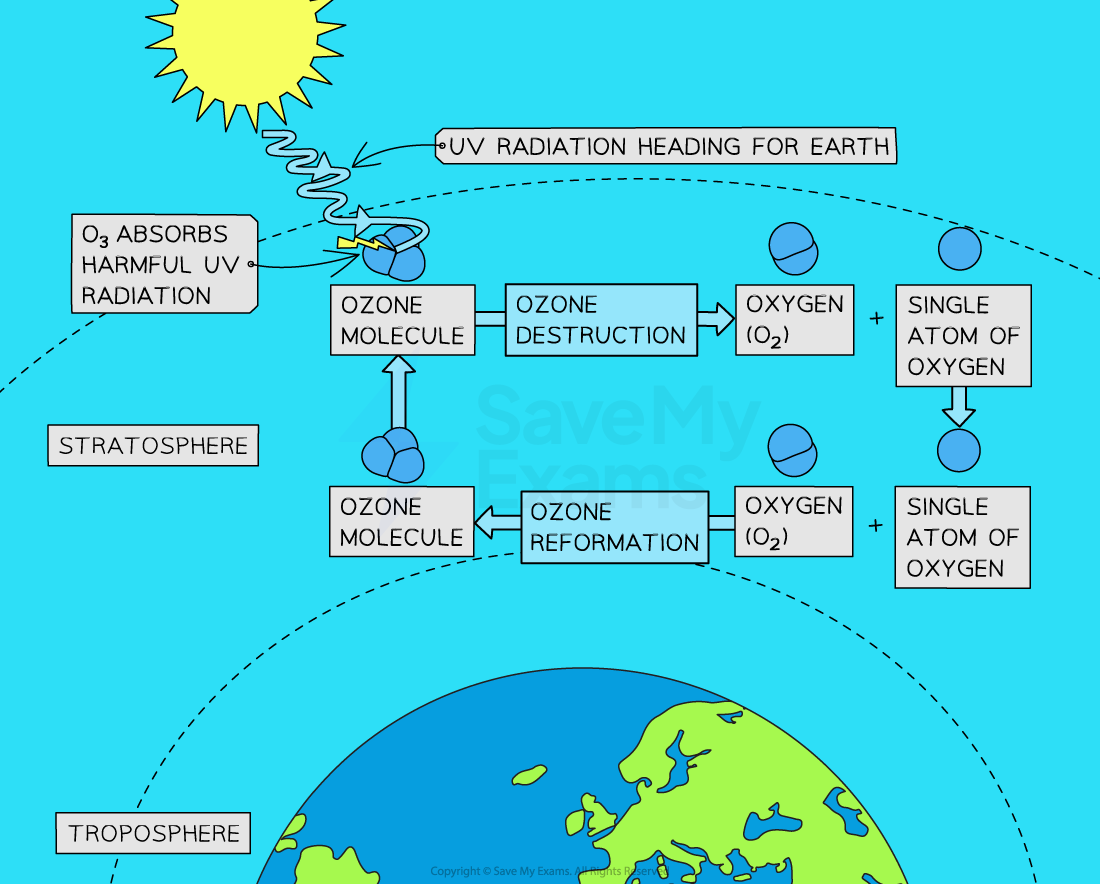
Ozone Destruction & Reformation
When UV radiation from the Sun interacts with ozone, some of the ozone molecules absorb the energy and break apart, resulting in the formation of an oxygen molecule (O₂) and a free oxygen atom (O)
This process of ozone destruction occurs naturally in the stratosphere due to the presence of UV radiation
However, under normal conditions, the free oxygen atom (O) can combine with another oxygen molecule (O₂) to form ozone (O₃) again
This ozone destruction and reformation creates a dynamic equilibrium in the stratosphere, where there is a continuous cycle of ozone molecules being broken apart and reformed
This dynamic equilibrium ensures that the concentration of ozone in the stratosphere remains relatively stable over time, as the rate of the forward reaction equals the rate of the backward reaction in the system, so the concentrations of the reactants and products remain relatively constant

Unlock more, it's free!
Did this page help you?