Reactions of Alcohols (OCR AS Chemistry A): Revision Note
Exam code: H032
Combustion of alcohols
Alcohols react with oxygen in the air when ignited and undergo complete combustion to form carbon dioxide and water
alcohol + oxygen → carbon dioxide + water

Complete combustion of alcohols to produce carbon dioxide and water
Lower alcohols burn with an almost invisible flame and make good fuels
Ethanol can be produced sustainably as a fuel by the fermentation of sugars
Energy density is the amount of energy released in kJ per kg of fuel
The energy density of ethanol is lower than gasoline
This means that ethanol fuelled cars need larger tanks or more frequent refuelling
Blending ethanol with petrol or diesel:
Increases the energy density
Makes flames more visible in fires, improving safety
There are socio-economic concerns about using farm land to grow fuel crops
Some argue that the land should be used for food production
Oxidation of alcohols
Primary alcohols can be oxidised to form aldehydes and then further oxidised to carboxylic acids
Secondary alcohols can be oxidised to form ketones only
Tertiary alcohols do not undergo oxidation
Oxidising agents
Common oxidising agents for alcohols include:
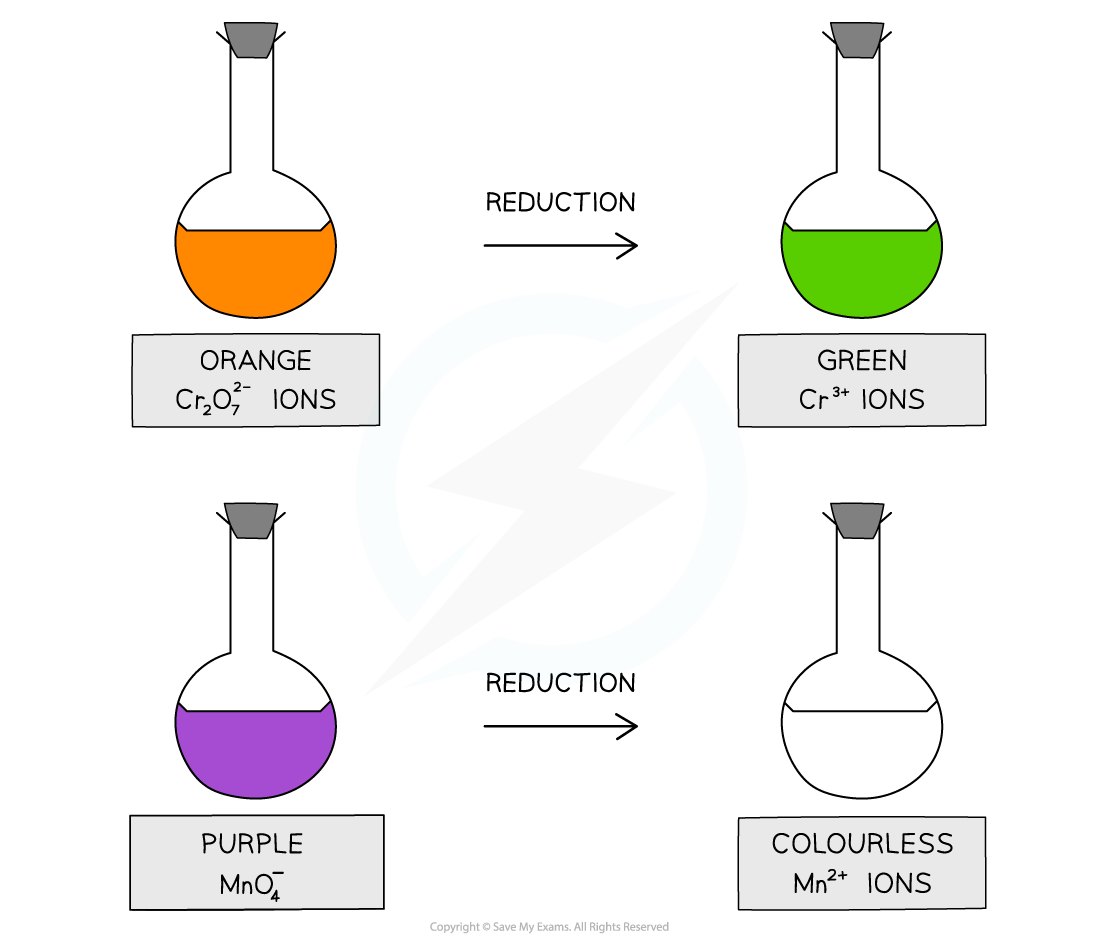
Acidified potassium dichromate(VI) (K2Cr2O7) – orange solution
Acidified potassium manganate(VII) (KMnO4) – purple solution
When alcohols are oxidised:
Orange Cr2O72- ions are reduced to green Cr3+ ions
Purple MnO4- ions are reduced to colourless Mn2+ ions
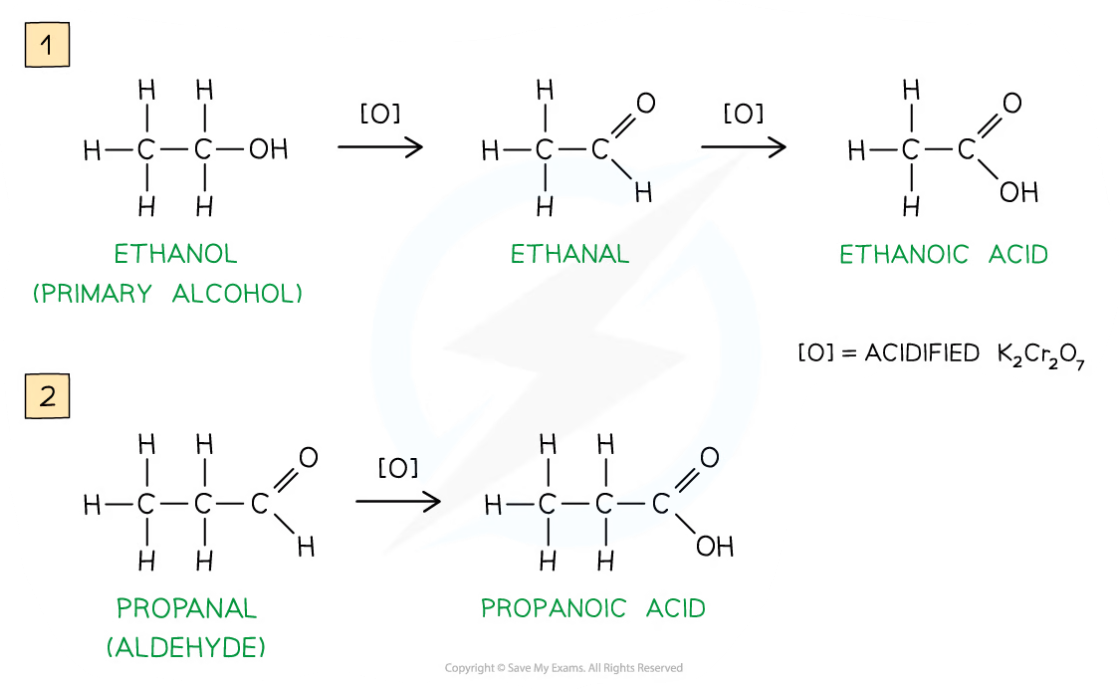
Forming aldehydes and carboxylic acids
Aldehyde formation
A primary alcohol is gently heated with oxidising agent
This forms an aldehyde
The aldehyde formed has a lower boiling point than the alcohol
So, the aldehyde can be distilled off as it forms
Carboxylic acid formation
If the aldehyde is not distilled off, refluxing with excess oxidising agent produces a carboxylic acid

Forming ketones
A secondary alcohol is gently heated with oxidising agent
This forms a ketone

Since ketones cannot be further oxidised, there is no need to distil off the product during the reaction
Elimination & substitution reactions of alcohols
Elimination reaction of alcohols
Alcohols can also undergo dehydration to form alkenes
This is called an elimination reaction
These elimination reactions produce:
The desired alkene
Water as a small molecule byproduct
The water is formed from the -OH group and a hydrogen atom on the adjacent carbon atom
Common reaction conditions include:
Passing the alcohol vapour over a hot catalyst of aluminium oxide (Al2O3) powder or pieces of porous pot
Heating the alcohol with excess hot, concentrated sulfuric acid or phosphoric acid catalyst

Substitution reactions of alcohols
In substitution reactions, a hydroxy group (-OH) of the alcohol is replaced by a halogen
This forms a haloalkane
The halogen can be made in situ during the reaction:
For example, KBr with H2SO4 or H3PO4 produces HBr, which reacts with the alcohol to form the haloalkane


Unlock more, it's free!
Did this page help you?