Polymers from Alkenes (OCR AS Chemistry A): Revision Note
Exam code: H032
Addition Polymerisation
Addition polymerisation is one of the most important addition reactions of alkenes which form the basis of the plastic industry
Addition polymerisation is the reaction in which many monomers containing at least one C=C double bond form long chains of polymers as the only product
Just like in other addition reactions of alkenes, the π-bond in each C=C bond breaks and then the monomers link together to form new C-C single bonds
A polymer is a long-chain molecule that is made up of many repeating units
The small, reactive molecules that react together to form the polymer are called monomers
A polymerisation reaction can be represented by a general formula or by using displayed formulae
E.g. poly(ethene) and poly(chloroethene) (also known as PVC) are polymers made up of the ethene and chloroethene monomers respectively and are commonly used in making plastics

The general formulae of the addition polymerisation of ethene (1) and chloroethene (2)
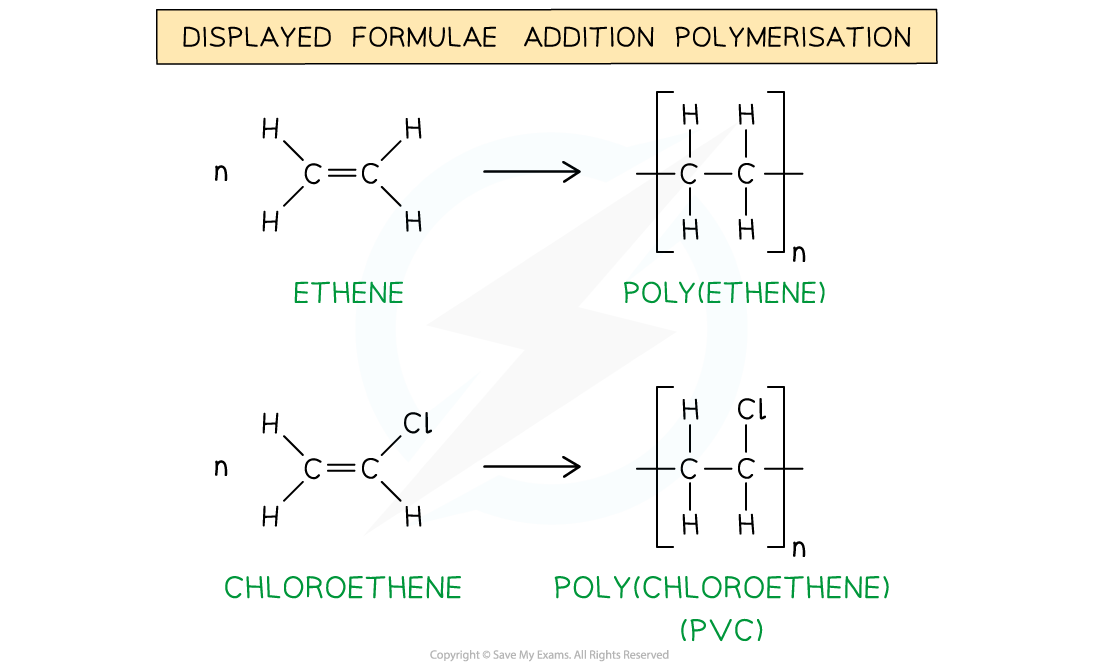
The addition polymerisation of ethene (1) and chloroethene (2)
Just like any other addition reaction of alkenes, addition polymerisation gives only one product
Deducing repeat units
A repeat unit is the smallest group of atoms that when connected one after the other make up the polymer chain
It is represented by square brackets in the displayed and general formula
In poly(alkenes) (such as poly(ethene)) and substituted poly(alkenes) (such as PVC) made of one type of monomer the repeating unit is the same as the monomer except that the C=C double bond is changed to a C-C single bond

The repeating units of poly(ethene) and poly(chloroethene) are similar to their monomer except that the C=C bond has changed into a C-C bond
Worked Example
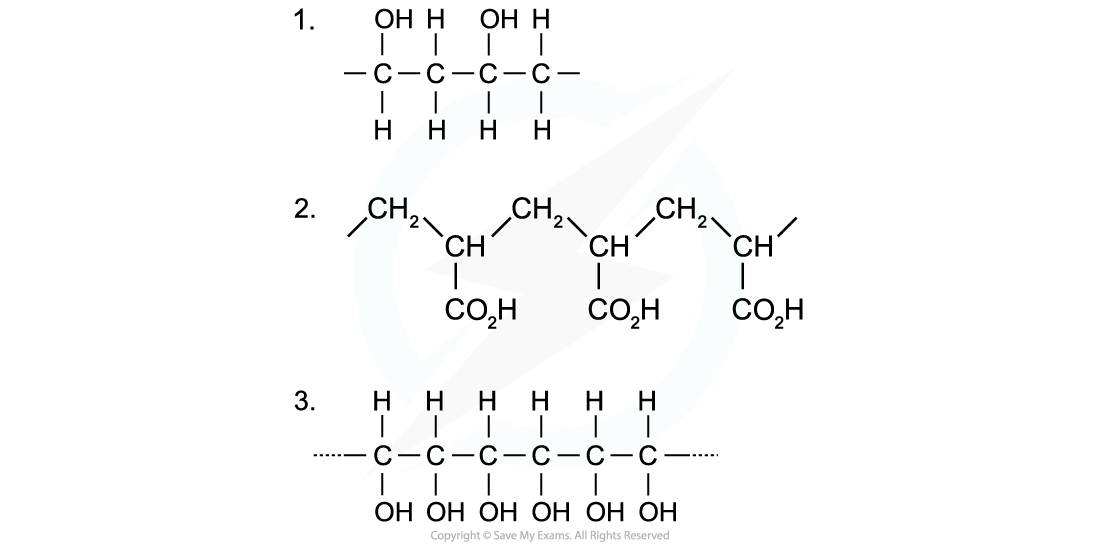
Identify the monomers present in the given sections of addition polymer molecules:
Answers
Answer 1:
When ethenol (CH(OH)=CH2) is polymerised, the C-C double bond opens to produce a repeating unit of CH(OH)-CH2. This gives the polymer poly(ethenol)

Answer 2:
To find the monomer, first the repeating unit should be deduced. Repeating units have only 2 carbons in the polymer main chain

Since the repeating unit is now found, it can be concluded that the monomer is prop-2-enoic acid

Answer 3:
Again, the repeating unit only has 2 carbons in the polymer chain which in this case are two carbon atoms that each contain one OH group
Thus, when ethene-1,2-diol (CH(OH)=CH(OH)) is polymerised, the C=C double bond opens to produce a repeating unit of CH(OH)-CH(OH) which gives the polymer poly(ethene-1,2-diol)

Examiner Tips and Tricks
The section of the polymer chain shown inside the square brackets by the structural or displayed formula is the repeat unit and not the monomer
The monomer is the same as the repeat unit except for that it has C=C bonds instead of C-C bonds
Waste polymers & Alternatives
Polymers provide a readily available, cheap alternative to many metal, glass, paper and cardboard materials in everyday use
The low reactivity of many polymers makes them ideal for certain uses, e.g. food packaging, but at the same time creates problems with their disposal as a lot of polymers are non-biodegradable
There are many published articles about the environmental problems of waste plastic killing marine animals
One method of polymer disposal continues to be the use of landfill sites
This is not ideal and various initiatives are being introduced aiming to reduce this method of waste disposal in general as well as with specific regard to polymers
Recycling
Polymer recycling reduces the amount of waste that it going to landfill sites
Newer landfill sites can have a recycling point where the new waste is brought before going to into the actual landfill - this is in an effort to reduce the amount of polymers (and other recyclable materials) unnecessarily going into the waste site
The recycling of polymers can also reduce the use of finite resources
Lots of polymers are made from the products of cracking crude oil and it's fractions
Recycling polymers is a time-consuming process as they have to be sorted into the different categories
These categories are usually shown somewhere on the plastic / polymer product with the recycling symbol and numbers or abbreviations for the different polymers, e.g.:

The recycling symbol for the polyethylene terephthalate polymer
After sorting, the polymers are chopped, washed, dried, melted and then cast into pellets ready for use
However, mixed polymers can mean that this process is wasted as its produces an unusable mix of polymers
Certain polymers can cause problems when recycling due to their chemical composition, e.g. PVC contains a large amount of toxic chlorine which can be released
Modern techniques are overcoming this PVC problem by dissolving the polymer and precipitating out the recycled material
Combustion
Some petroleum / natural gas derived polymers are still difficult to recycle
Since they have a large amount of energy stored within the polymer chains, these polymers can be incinerated
This process can then be used to boil water and use the water vapour to turn turbines inside a power station, in a similar fashion to coal-fired power stations
This process still causes environmental pollution as the carbon within the polymer can be released as carbon dioxide contributing to global warming
Other toxic waste products include hydrogen chloride from the combustion of PVC
Feedstock recycling
Feedstock recycling is where waste polymers are broken down, by chemical and thermal processes, into monomers, gases and oils
These products are then used as the raw materials in the production of new polymers and other organic chemicals
The major benefit of feedstock recycling, compared to other methods of polymer disposal, is that it works with unsorted and unwashed polymers
Bioplastics
Bioplastics are polymers that are made from plant starch / cellulose, plant oils and plant proteins
They provide a renewable and sustainable alternative to the current polymers which are predominantly based on finite resources such as crude oil
Biodegradable polymers
Biodegradable polymers can be broken down over time by microorganisms
Common products from this process include carbon dioxide, water and other organic compounds
The polyester and polyamide condensation polymers are considered to be biodegradable as they can be broken down using hydrolysis reactions
This is a major advantage over the polymers produced using alkene monomers (polyalkenes)
When polyesters and polyamides are taken to landfill sites, they can be broken down easily and their products used for other applications
Compostable polymers
Compostable polymers are commonly plant based
Plant starch is being used in the production of biodegradable bin liners
Sugar cane fibres are replacing polystyrene in the production of disposable plates and cups
Compostable polymers degrade naturally leaving no harmful residues
Photodegradable polymers
Photodegradable polymers contain bonds that are weakened by absorbing light / visible radiation
This starts the breakdown of the polymer
A lot of photodegradable polymers are oil-based
In certain cases, an additive that absorbs light is mixed into the polymer to promote degradation

Unlock more, it's free!
Did this page help you?