Electrophilic Addition (OCR AS Chemistry A): Revision Note
Exam code: H032
Electrophilic Addition
Electrophilic addition is the addition of an electrophile to an alkene double bond, C=C
The alkene double bond, C=C, is an area of high electron density which makes it susceptible to attack by electrophiles
The C=C bond breaks forming a single C-C bond and 2 new bonds from each of the two carbon atoms
Electrophilic addition of hydrogen halides
A hydrogen halide molecule is polar as the hydrogen and halogen atoms have different electronegativities
For example, in a molecule of hydrogen bromide, HBr, the bromine atom has a stronger pull on the electrons in the H-Br bond
As a result of this, the Br atom has a partial negative and the H atom a partial positive charge

Due to differences in electronegativities of the hydrogen and bromine atom, HBr is a polar molecule
In electrophilic addition reactions with hydrogen halides, the H atom acts as an electrophile by accepting a pair of electrons from the C=C bond in the alkene
The H-Br bond breaks heterolytically, forming a Br- ion
This results in the formation of a highly reactive carbocation intermediate which reacts with the bromide ion, Br-
For example, the mechanism for the electrophilic addition of hydrogen bromide and ethene is:
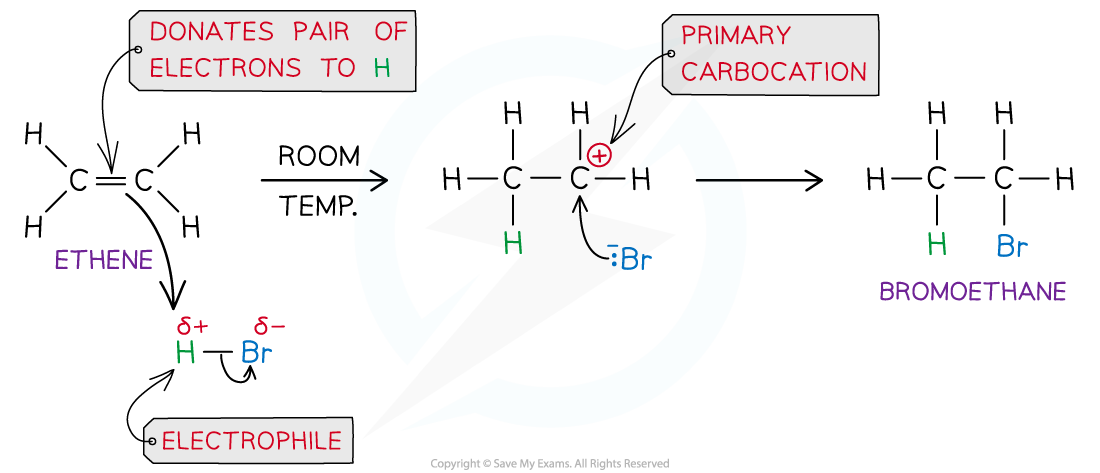
Electrophilic addition reaction of HBr and ethene to form bromoethane
Examiner Tips and Tricks
For electrophilic addition mechanisms, the curly arrows must:
Be double-headed to show the movement of a pair of electrons
Start from a lone pair of electrons or an area of high electron density, e.g. the C=C bond
Move towards a δ+ electrophile or the positive charge of a carbocation
Examiners often comment about the poor and incorrect use of curly arrows in organic mechanisms
Electrophilic addition of halogens
The mechanism for the electrophilic addition of halogens (and hydrogen) is the same as the electrophilic addition of hydrogen halides with one key exception:
Hydrogen halide molecules have a permanent dipole (as shown above)
Halogen molecules have a temporary (or induced) dipole caused by the repulsion of the halogens electrons by the high electron density C=C bond

The temporary (or induced) dipole in a halogen molecule
Markownikoff Addition
Carbocations are positively charged carbon atoms with only three covalent bonds instead of four
There are three types of carbocations: primary, secondary and tertiary
Inductive effect
The alkyl groups attached to the positively charged carbon atoms are ‘electron donating groups’
This is also known as the inductive effect of alkyl groups
The inductive effect is illustrated by the use of arrowheads on the bonds to show the alkyl groups pushing electrons towards the positively charged carbon
This causes the carbocation to become less positively charged
As a result of this, the charge is spread around the carbocation which makes it energetically more stable
This means that tertiary carbocations are the most stable as they have three electron-donating alkyl groups which energetically stabilise the carbocation
Due to the positive charge on the carbon atom, carbocations are electrophiles
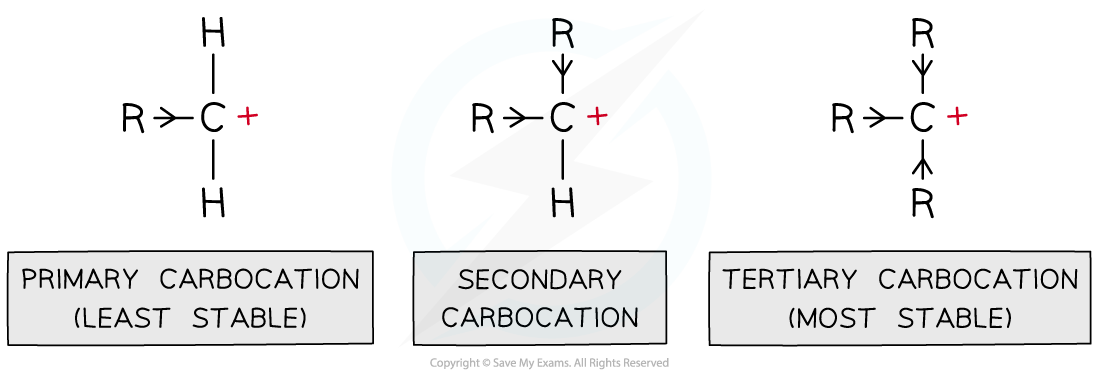
Alkyl groups push electron density towards the carbocation making it energetically more stable; the more alkyl groups the carbocation is bonded to, the more stabilised it is
Markownikoff’s rule
Markownikoff’s rule predicts the outcome of electrophilic addition reactions and states that:
In an electrophilic addition reaction of a hydrogen halide (HX) to an alkene, the halogen ends up bonded to the most substituted carbon atom
In an electrophilic addition reaction of an interhalogen to an alkene, the most electronegative halogen ends up bonded to the most substituted carbon atom
Markownikoff addition applies to electrophilic addition reactions with unsymmetrical alkanes, e.g. propene and but-1-ene
Markownikoff addition favours the formation of the major product
Anti-Markownikoff addition favours the formation of the minor product
In electrophilic addition reactions, an electrophile reacts with the double bond of alkenes (as previously discussed)
The mechanism for electrophilic addition reactions with unsymmetrical alkenes is slightly different, e.g. propene + hydrogen bromide

The electrophile reacts with the electron-rich C-C double bond
The electrophile can attach in two possible ways:
Breaking the C=C bond and attaching to the least substituted carbon
This will give the most stable carbocation as an intermediate that will form the major product
Breaking the C=C bond and attaching to the most substituted carbon
This will give the least stable carbocation as an intermediate that will form the minor product

The major and minor carbocation intermediates formed during the reaction of propene and hydrogen bromide
The nucleophile will bond to the positive carbon atom of the carbocation
The more stable carbocation produces the major product
The less stable carbocation produces the minor product

Formation of the major and minor products of the reaction of propene with hydrogen bromide
The mechanism for the electrophilic addition of hydrogen bromide to propene, showing the formation of the major and minor products can be shown as:

The electrophilic addition reaction mechanism of HBr and propene to form 1-bromopropane and 2-bromopropane
Examiner Tips and Tricks
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
When more than one carbocation can be formed, the major product of the reaction will be the one that results from the nucleophilic attack of the most stable carbocation.

Unlock more, it's free!
Did this page help you?