Hess Cycles (Edexcel AS Chemistry) : Revision Note
Constructing Hess Cycles
In 1840, the Russian chemist Germain Hess formulated a law which went on to be known as Hess’s Law
This went on to form the basis of one of the laws of thermodynamics. The first law of thermodynamics relates to the Law of Conservation of Energy
It is sometimes expressed in the following form:
Energy cannot be created or destroyed, it can only change form
This means that in a closed system, the total amount of energy present is always constant
Hess’s law can be used to calculate the standard enthalpy change of a reaction from known standard enthalpy changes
Hess’s Law states that:
"The total enthalpy change in a chemical reaction is independent of the route by which the chemical reaction takes place as long as the initial and final conditions are the same."
This means that whether the reaction takes place in one or two steps, the total enthalpy change of the reaction will still be the same
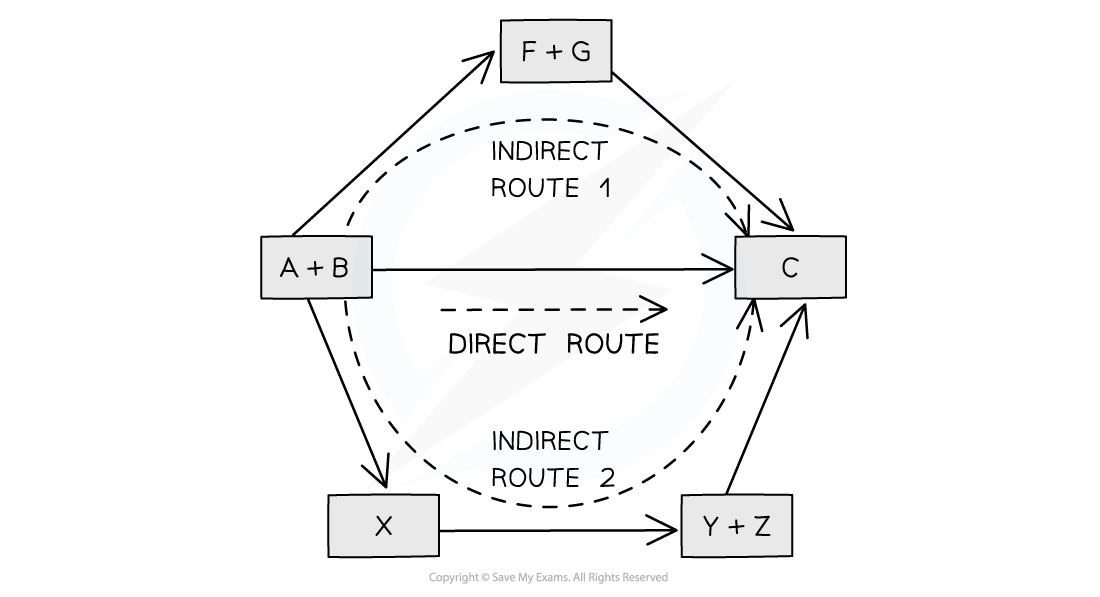
The diagram above illustrates Hess’ Law: the enthalpy change of the direct route, going from reactants (A+B) to product (C) is equal to the enthalpy change of the indirect routes
Hess’ Law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, e.g.:
3C (s) + 4H2 (g) → C3H8(g)
ΔfH (propane) can’t be found experimentally as hydrogen and carbon don’t react under standard conditions
Calculating ΔrH from ΔfH using Hess’s Law energy cycles
You can see the relationships on the following diagram:

The enthalpy change from elements to products (direct route) is equal to the enthalpy change of elements forming reactants and then products (indirect route)
The products can be directly formed from the elements = ΔH2
OR
The products can be indirectly formed from the elements = ΔH1 + ΔHr
Equation
ΔH2 = ΔH1 + ΔHr
Therefore for energy to be conserved,
ΔHr = ΔH2 – ΔH1
Examiner Tips and Tricks
You do not need to learn Hess's Law word for word as it is not a syllabus requirement, but you do need to understand the principle as it provides the foundation for all the problem solving in Chemical Energetics
Hess Cycle Calculations
Hess cycles can be used to calculate various enthalpy changes as long as sufficient information about the other sides of the cycle is known
Worked Example
Calculating the enthalpy change of reaction
Calculate the ΔHf for the following reaction:
2NaHCO3 (s) → Na2CO3 (s) + CO2 (g) + H2O (I)
The table below shows the standard enthalpy of formations (ΔHfꝋ) relevant to this reaction:

Answer
Step 1: Write the balanced equation at the top

Step 2: Draw the cycle with the elements at the bottom
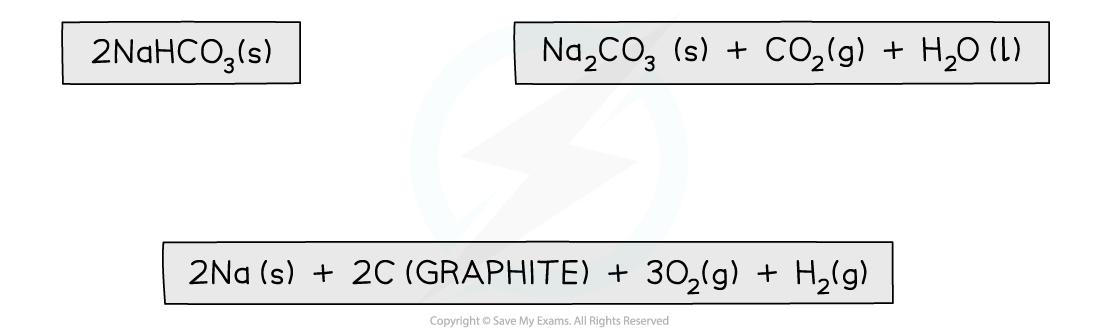
Step 3: Draw in all arrows, making sure they go in the correct directions. Remember: You are working with enthalpies of formation which means that the arrows go up to show the compounds being made from the elements. Write the standard enthalpy of formations

Step 4: Apply Hess’s Law
ΔrH = ΔH2 - ΔH1
It is minus ΔH1 because you have to go in the opposite direction of the arrow
ΔH2 is ΔfH [Na2CO3 (s)] + ΔfH [CO2 (g)] + ΔfH [H2O (l)]
ΔH1 is 2 x ΔfH [NaHCO3 (s)]
ΔrH = (ΔfH [Na2CO3 (s)] + ΔfH [CO2 (g)] + ΔfH [H2O (l)]) - (2ΔfH [NaHCO3 (s)])
ΔrH = ((-1130.7) + (-393.5) + (-285.8)) - (2 x (-950.8))
ΔrH = +91.6 kJ mol-1
Examiner Tips and Tricks
Keep your enthalpy values inside their own brackets so that you don't accidentally lose a minus sign

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
