Error & Uncertainty (Edexcel AS Chemistry): Revision Note
Error & Uncertainty
An error is the difference between a value or quantity obtained in an experiment and an accepted or literature value for an experiment
There are two types of errors in experiments, random errors and systematic errors
Random Errors
When you are reading an instrument and estimate the final digit, there is an equal chance that you may read it slightly too high or slightly too low
This is a random error
Random errors are can be affected by:
How easily the instrument or scale is to read
The person reading the scale poorly
Changes in the environment, for example
fluctuations in the temperature of the lab
air currents in the room
Random errors will pull a result away from an accepted value in either direction (either too high or too low)
Repeating the experiment and working with the mean average of the results can help to reduce the effects of random errors
Systematic Errors
Systematic errors are errors that occur as a result of a faulty or poorly designed experimental procedure
Systematic errors will always pull the result away from the accepted value in the same direction (always too high or always too low)
For example,
If you forget to zero an electronic balance (using the tare button) the mass weighings will always be higher than they should be
If you don't read the volume in a burette at eye level, the volumes will always be smaller than they should be due to a parallax error
If you fail to keep a cap on a spirit burner in a calorimetry experiment, the alcohol will evaporate and give you a larger mass loss
Repeating the experiment and working with the average value will not remove any systematic errors
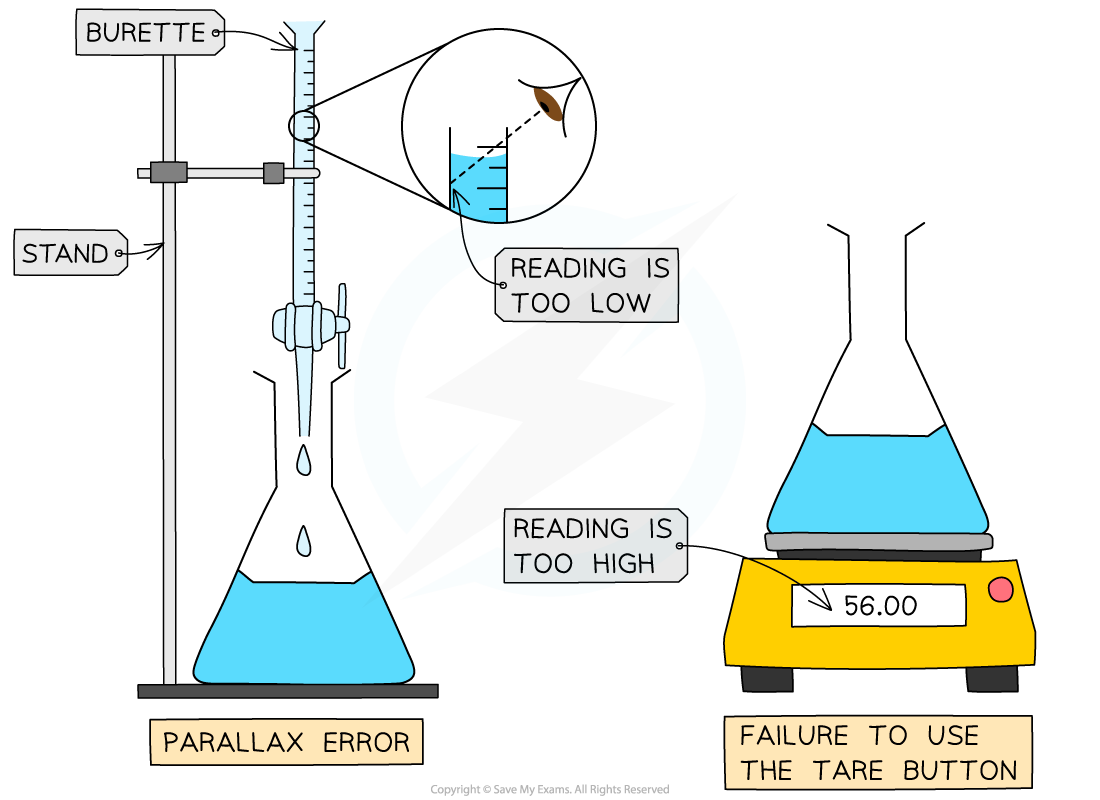

Systematic errors
Percentage Uncertainties
Percentage uncertainties are a way to compare the significance of an absolute uncertainty on a measurement
This is not to be confused with percentage error, which is a comparison of a result to a literature value
The formula for calculating percentage uncertainty is as follows:
Percentage uncertainty =
Some examples of percentage uncertainty calculations for common laboratory apparatus:


Calculating Percentage Uncertainty
Adding or subtracting measurements
When you are adding or subtracting two measurements then you add together the absolute measurement uncertainties
For example,
Using a balance to measure the initial and final mass of a container
Using a thermometer for the measurement of the temperature at the start and the end
Using a burette to find the initial reading and final reading
In all these examples you have to read the instrument twice to obtain the quantity
If each you time you read the instrument the measurement is 'out' by the stated uncertainty, then your final quantity is potentially 'out' by twice the uncertainty
Multiplying or dividing measurements
When you multiply or divide experimental measurements then you add together the percentage uncertainties
You can then calculate the absolute uncertainty from the sum of the percentage uncertainties
Examiner Tips and Tricks
If you are multiplying or dividing data you should quote the answer to the same number of significant figures as the least precise data.
When you add or subtract data you should use the same number of decimal places as the least precise data value

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
