Periodicity (Edexcel AS Chemistry): Revision Note
Exam code: 8CH0
Melting & Boiling Point Trends
Elements in the periodic table are arranged in order of increasing atomic number and placed in vertical columns (groups) and horizontal rows (periods)
The elements across the periods show repeating patterns in chemical and physical properties
This is called periodicity
The Periodic Table
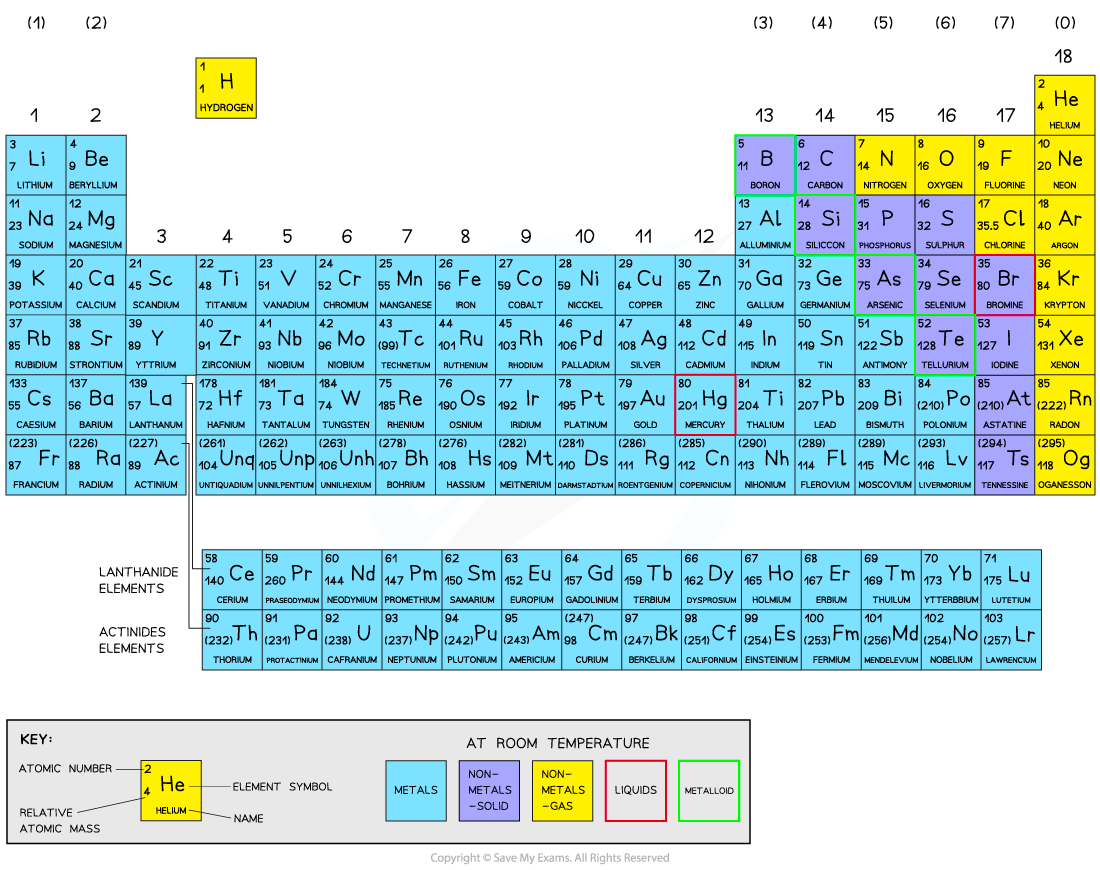
All elements are arranged in the order of increasing atomic number from left to right
Melting point
Period 2 and 3 elements follow the same pattern in relation to their melting points
Melting points of the elements across Period 3 table


Ions of Period 3 elements with increasing positive charge (metals) and increasing of number of outer electrons across the period
A general increase in melting point for the Period 3 elements up to silicon is observed
Silicon has the highest melting point
After the Si element the melting points of the elements decreases significantly
The above trends can be explained by looking at the bonding and structure of the elements
Bonding & structure of the elements table

The table shows that Na, Mg and Al are metallic elements which form positive ions arranged in a giant lattice in which the ions are held together by a 'sea' of delocalised electrons

Metal cations form a giant lattice held together by electrons that can freely move around
The electrons in the ‘sea’ of delocalised electrons are those from the valence shell of the atoms
Na will donate one electron into the ‘sea’ of delocalised electrons, Mg will donate two and Al three electrons
As a result of this, the metallic bonding in Al is stronger than in Na
This is because the electrostatic forces between a 3+ ion and the larger number of negatively charged delocalised electrons is much larger compared to a 1+ ion and the smaller number of delocalised electrons in Na
Because of this, the melting points increase going from Na to Al
Si has the highest melting point due to its giant molecular structure in which each Si atom is held to its neighbouring Si atoms by strong covalent bonds
P, S, Cl and Ar are non-metallic elements and exist as simple molecules (P4, S8, Cl2 and Ar as a single atom)
The covalent bonds within the molecules are strong, however, between the molecules, there are only weak instantaneous dipole-induced dipole forces
It doesn’t take much energy to break these intermolecular forces
Therefore, the melting points decrease going from P to Ar (note that the melting point of S is higher than that of P as sulphur exists as larger S8 molecules compared to the smaller P4 molecule)
Atomic radius
The atomic radius is the distance between the nucleus and the outermost electron of an atom
The atomic radius is measured by taking two atoms of the same element, measuring the distance between their nuclei and then halving this distance
In metals this is also called the metallic radius and in non-metals, the covalent radius
Atomic radii of period 3 elements
You can see a clear trend across the period which also repeated in period 2


The graph shows a decrease in atomic radii of period 3 elements across the period
Across the period, the atomic radii decrease
This is because the number of protons (the nuclear charge) and the number of electrons increases by one every time you go an element to the right
The elements in a period all have the same number of shells (so the shielding effect is the same)
This means that as you go across the period the nucleus attracts the electrons more strongly pulling them closer to the nucleus
Because of this, the atomic radius (and thus the size of the atoms) decreases across the period
Ionisation Energy Trends
Ionisation energy across period 2 and 3
The ionisation energy across a period generally increases due to the following factors:
Across a period the nuclear charge increases
This causes the atomic radius of the atoms to decrease, as the outer shell is pulled closer to the nucleus, so the distance between the nucleus and the outer electrons decreases
The shielding by inner shell electrons remain reasonably constant as electrons are being added to the same shell
It becomes harder to remove an electron as you move across a period; more energy is needed
So, the ionisation energy increases
Dips in the trend for period 2
There is a slight decrease in IE1 between beryllium and boron as the fifth electron in boron is in the 2p subshell, which is further away from the nucleus than the 2s subshell of beryllium
Beryllium has a first ionisation energy of 900 kJ mol-1 as its electron configuration is 1s2 2s2
Boron has a first ionisation energy of 800 kJ mol-1 as its electron configuration is 1s2 2s2 2px1
There is a slight decrease in IE1 between nitrogen and oxygen due to spin-pair repulsion in the 3px orbital of oxygen
Nitrogen has a first ionisation energy of 1400 kJ mol-1 as its electron configuration is 1s2 2s2 2px1 2py1 2pz1
Oxygen has a first ionisation energy of 1310 kJ mol-1 as its electron configuration is 1s2 2s2 2px2 2py1 2pz1
In oxygen, there are 2 electrons in the 2px orbital, so the repulsion between those electrons makes it slightly easier for one of those electrons to be removed
Dips in the trend for period 3
There is again a slight decrease between magnesium and aluminium as the thirteenth electron in aluminium is in the 3p subshell, which is further away from the nucleus than the 3s subshell of magnesium
Magnesium has a first ionisation energy of 738 kJ mol-1 as its electron configuration is 1s2 2s2 2p6 3s2
Aluminium has a first ionisation energy of 578 kJ mol-1 as its electron configuration is 1s2 2s2 2p6 3s2 3px1
There is a slight decrease in IE1 between phosphorus and sulfur due to spin-pair repulsion in the 3px orbital of sulfur
Phosphorus has a first ionisation energy of 1012 kJ mol-1 as its electron configuration is 1s2 2s2 2p6 3s2 3px1 3py1 3pz1
Sulfur has a first ionisation energy of 1000 kJ mol-1 as its electron configuration is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1
In sulfur, there are 2 electrons in the 3px orbital, so the repulsion between those electrons makes it slightly easier for one of those electrons to be removed

Unlock more, it's free!
Did this page help you?