Classifying & Testing for Alcohols (Cambridge (CIE) AS Chemistry)
Revision Note
Classifying Alcohols
Primary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to one other carbon atom (or alkyl group)
Secondary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to two other carbon atoms (or alkyl groups)
Tertiary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to three other carbon atoms (or alkyl groups)
Primary, secondary and tertiary alcohols

Classifying primary, secondary and tertiary alcohols and alcohols with more than one alcohol group
Only primary and secondary alcohols can get oxidised when mildly oxidised with acidified K2Cr2O7
Primary alcohols get mildly oxidised to aldehydes
Secondary alcohols get mildly oxidized to ketones
Tertiary alcohols do not undergo oxidation with acidified K2Cr2O7
Therefore, only the oxidation of primary and secondary alcohols will change the colour of K2Cr2O7 solution as the orange Cr2O72- ions are reduced to green Cr3+ ions
Testing for alcohols
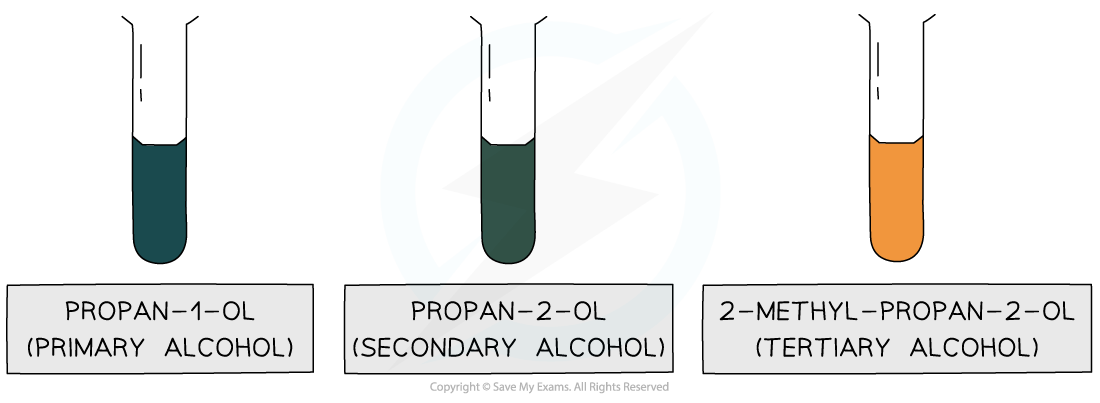
Only propan-1-ol and propan-2-ol, which are primary and secondary alcohols respectively, can get oxidised, turning the orange solution green; no colour change is observed with 2-methyl-propan-2-ol, which is a tertiary alcohol
Test for Alcohols
Tri-iodomethane (also called iodoform) forms a yellow precipitate with methyl ketones
Methyl ketones are compounds that have a CH3CO-group
Ethanal also contains a CH3CO- group and therefore also forms a yellow precipitate with iodoform
The reagent is heated with an alkaline solution of iodine
This reaction involves a halogenation and hydrolysis step
In the halogenation step, all three H-atoms in the -CH3 (methyl) group are replaced for iodine atoms, forming -CI3
The intermediate compound is hydrolysed by alkaline solution to form a sodium salt (RCO2- Na+) and a yellow precipitate of CHI3
The iodoform reaction

The reaction of methyl ketones with iodoform results in the formation of a yellow CHI3 precipitate
Iodoform & alcohols
The position of a secondary alcohol can be deduced by reacting the compound with alkaline I2
If the -OH group is on the carbon atom next to a methyl group, it will firstly get oxidised to CH3CH(OH)- by the alkaline solution
This will result in the formation of a methyl ketone RCOCH3
The methyl ketone will then first get halogenated and then hydrolysed to form the sodium salt and the yellow precipitate
If no yellow precipitate is formed, then this means that the secondary alcohol is not on a carbon next to a methyl group
Using the iodoform test

The secondary alcohol butan-2-ol will firstly get oxidised to the methyl ketone butanone which will form a yellow precipitate when reacted with alkaline I2

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
