Electrophilic Addition of Alkenes (Cambridge (CIE) AS Chemistry) : Revision Note
Electrophilic Addition of Alkenes
The double bond in alkenes is an area of high electron density (there are four electrons found in this double bond)
This makes the double bond susceptible to attack by electrophiles (electron-loving species)
An electrophilic addition is the addition of an electrophile to a double bond
Electrophilic addition of hydrogen bromide
A molecule of hydrogen bromide (HBr) is polar as the hydrogen and bromine atoms have different electronegativities
The bromine atom has a stronger pull on the electrons in the H-Br bond
As a result of this, the Br atom has a partial negative and the H atom a partial positive charge
Explaining the polarity of a HBr molecule

Due to differences in electronegativities of the hydrogen and bromine atoms, HBr is a polar molecule
In an addition reaction, the H atom acts as an electrophile and accepts a pair of electrons from the C-C bond in the alkene
The H-Br bond breaks heterolytically, forming a Br- ion
This results in the formation of a highly reactive carbocation intermediate which reacts with the bromide ion, Br-
For example, the mechanism for the electrophilic addition of hydrogen bromide and ethene is:
Electrophilic addition of HBr mechanism
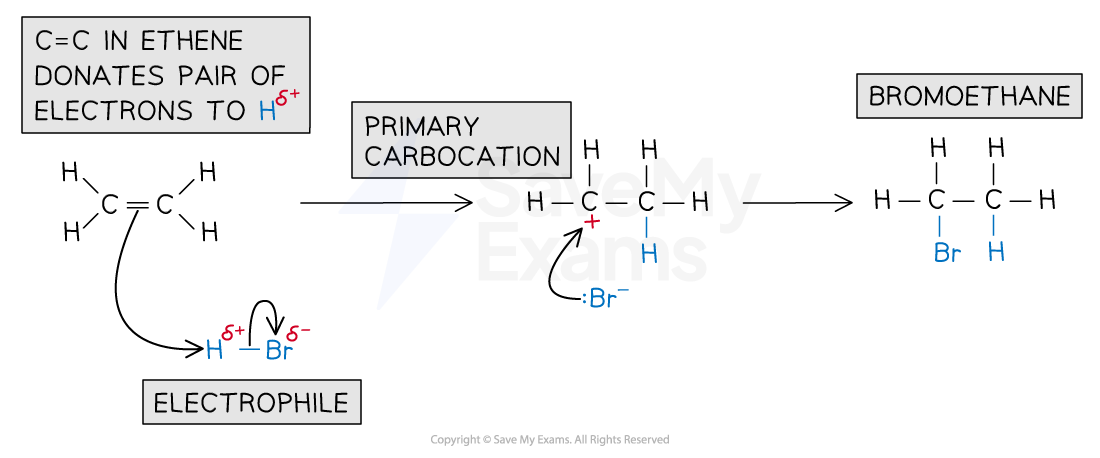
Electrophilic addition reaction of HBr and ethene to form bromoethane
Electrophilic addition of bromine
Bromine (Br2) is a non-polar molecule as both atoms have similar electronegativities and therefore equally share the electrons in the covalent bond
However, when a bromine molecule gets closer to the double bond of an alkene, the high electron density in the double bond repels the electron pair in Br-Br away from the closest Br atom
As a result of this, the closest Br atom to the double bond is slightly positive and the further Br atom is slightly negatively charged
The polarity of a Br2 molecule

Br2 is a non-polar molecule however when placed close to an area of high electron density it can get polarised
In an addition reaction, the closest Br atom acts as an electrophile and accepts a pair of electrons from the C-C bond in the alkene
The Br-Br bond breaks heterolytically, forming a Br- ion
This results in the formation of a highly reactive carbocation intermediate which reacts with the Br- (nucleophile)
Electrophilic addition of Br2 mechanism

Example of an electrophilic addition reaction of Br2 and ethene to form dibromoethane

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
