Chirality (Cambridge (CIE) AS Chemistry): Revision Note
Exam code: 9701
Chirality & Enantiomers
Chiral centres in non-cyclic molecules
A chiral centre in a molecule is a carbon atom that has four different atoms or groups of atoms attached
This gives rise to two optical isomers which are also called enantiomers
The enantiomers are mirror images of each other and cannot be superimposed
Chiral centres in non-cyclic molecules

When the molecule contains more than one chiral centre (asymmetric carbon) more than two optical isomers will be formed
If there are two chiral centres, each chiral centre will rotate the plane of polarised light clockwise and anticlockwise
There are four possible optical isomers
Molecules with multiple chiral centres

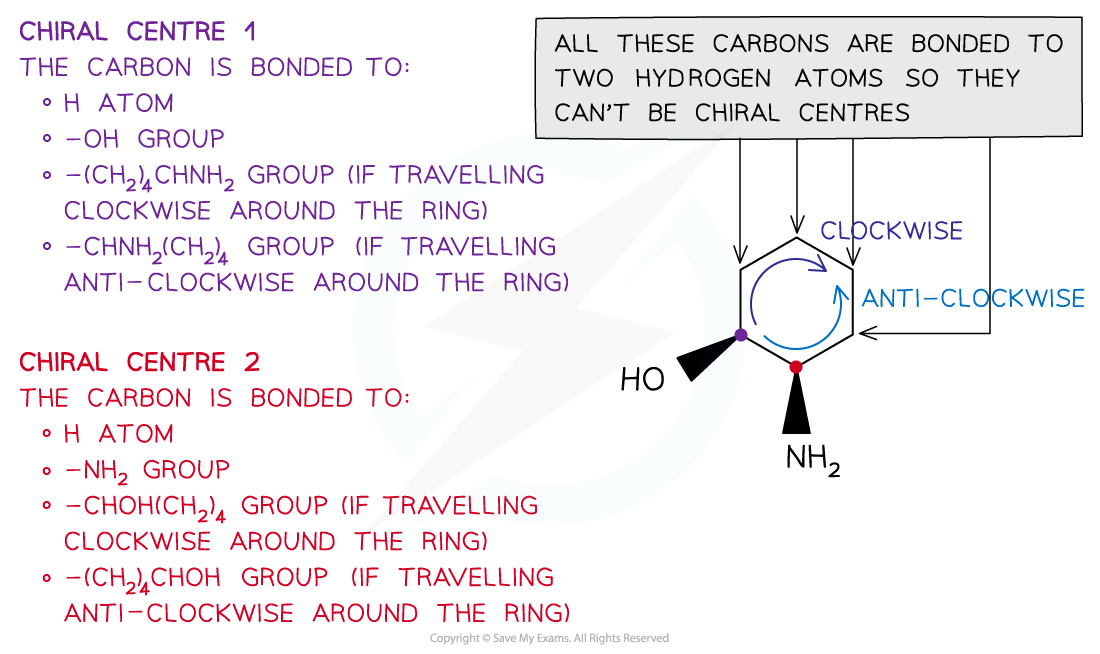
Chiral centres in cyclic molecules
To determine the chiral centre in a cyclic molecule, the carbon bonded to four different atoms or groups of atoms should be found
E.g. 2-aminocyclohexanol has two chiral centres so it can form four optical isomers
Chiral centres in cyclic molecules
Examiner Tips and Tricks
Use a molecular modelling kit and make the models of enantiomers to help you understand that the two molecules are non-superimposable and therefore non-identical
Identifying Chirality & Geometrical Isomerism
Identify chirality
Identifying chiral centres in cyclic and non-cyclic compounds is very straightforward as it is the carbon with four different atoms or atom groups in a molecule
This gives rise to two optical isomers
When more than two chiral centres are present, more than two optical isomers exist
The maximum number of stereoisomers that a molecule can have is 2n, where n is the number of chiral centres
So, a molecule with three chiral centres will have 23 = eight optical isomers
A molecule containing chiral centres is called a chiral molecule
Identifying geometrical isomers
Molecules with restricted rotation about the C-C bond can have geometrical isomers
This includes unsaturated and cyclic compounds
E.g. alkenes and cyclopentane
When the groups are positioned on the same side of the C-C double bond, the compound is a cis isomer
When the groups are positioned on opposite sides of the C-C double bond the compound is a trans isomer
Worked Example
Drawing optical isomers
Draw the optical isomers of the following compounds:

Answers:

Worked Example
Drawing geometrical isomers
Draw the geometrical isomers of the following compounds:

Answers:



Unlock more, it's free!
Did this page help you?