Oxidation of Alcohols (AQA AS Chemistry) : Revision Note
Oxidation of Alcohols
Oxidation of alcohols
Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
Secondary alcohols can be oxidised to form ketones only
Tertiary alcohols do not undergo oxidation
The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
Acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
Acidified means that the potassium dichromate(VI) is in a solution of dilute acid (such as dilute sulfuric acid)
For potassium dichromate(VI) to act as an oxidising agent, it itself needs to be reduced
This reduction requires hydrogen (H+) ions which are provided by the acidic medium
When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
Acidified potassium manganate(VII), KMnO4, is a purple oxidising agent
As with acidified K2Cr2O7 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
When alcohols are oxidised, the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
The primary alcohol is added to the oxidising agent and warmed
The aldehyde product has a lower boiling point than the alcohol reactant so it can be distilled off as soon as it forms
If the aldehyde is not distilled off, further refluxing with excess oxidising agent will oxidise it to a carboxylic acid
Since ketones cannot be further oxidised, the ketone product does not need to be distilled off straight away after it has been formed
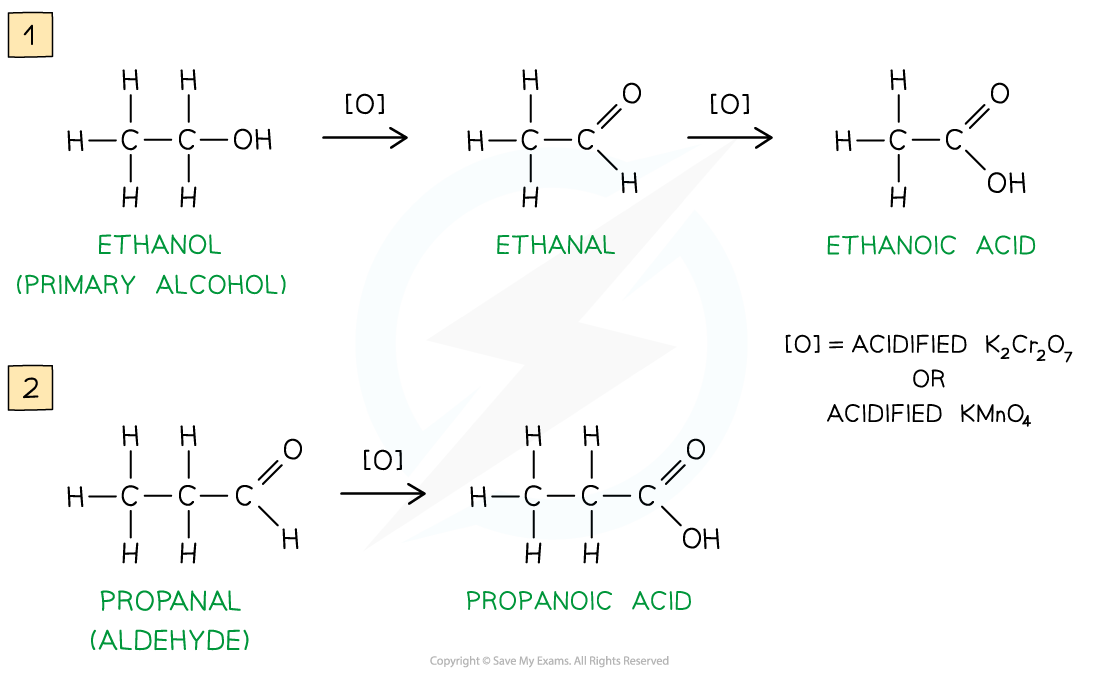
Oxidation Stages of Primary Alcohols

Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation

Further oxidation of the aldehyde via reflux can be done to produce a carboxylic acid

Oxidation of propan-2-ol by acidified K2Cr2O7 to form a ketone
Oxidation Products
Aldehydes and ketones are carbonyl compounds containing a C=O group
They can be prepared from the oxidation of primary and secondary alcohols respectively
Oxidising agents
The oxidising agents used to prepare aldehydes and ketones from alcohols include acidified potassium dichromate (K2Cr2O7) and acidified potassium manganate (KMnO4)
The acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
When the alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
The acidified potassium manganate(VII), KMnO4 is a purple oxidising agent
When the alcohols are oxidised the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
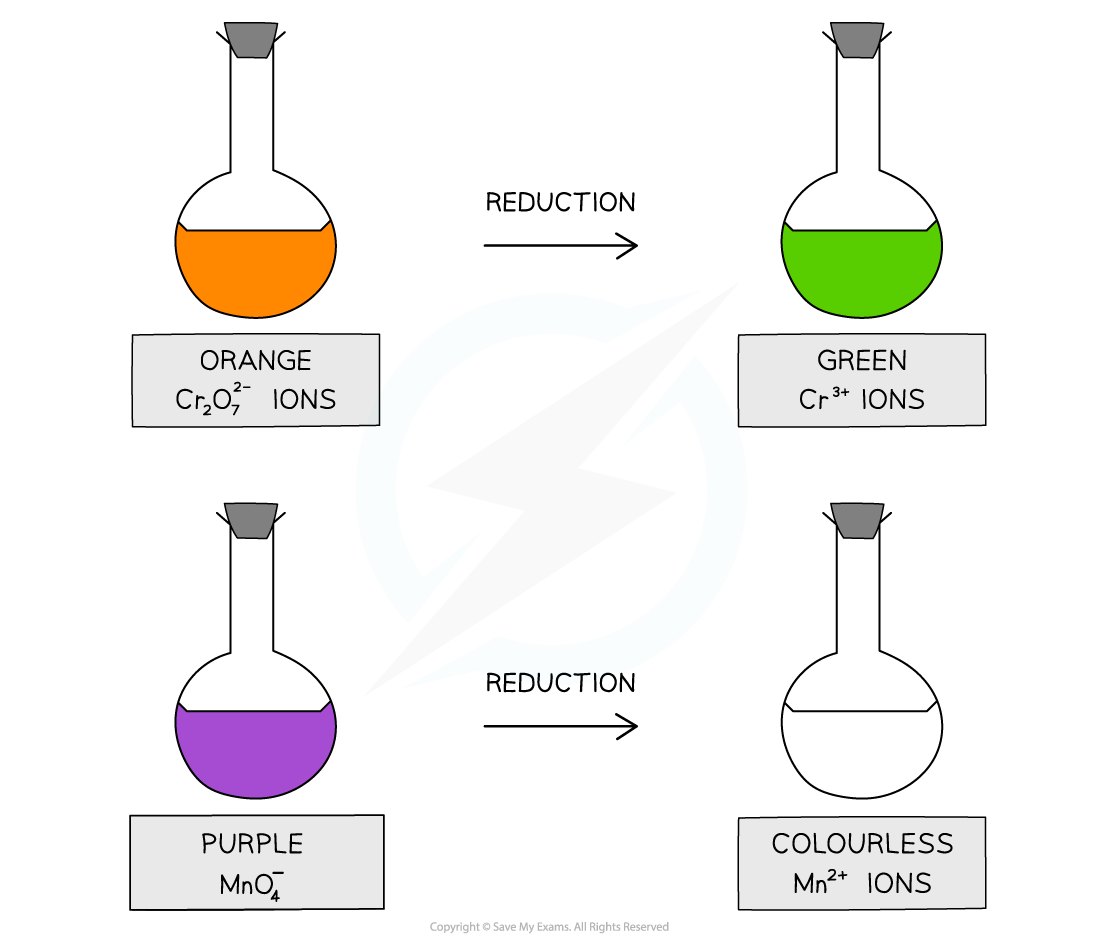
The oxidising agents change colour when they oxidise an alcohol and get reduced themselves
Testing for Oxidation Products
The presence of an aldehyde group (-CHO) in an unknown compound can be determined by the oxidising agents Fehling’s and Tollens’ reagents
Fehling’s solution
Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent
When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Cu2+ ions are reduced to Cu+ ions
In the alkaline conditions, the carboxylic acid formed will be neutralised to a carboxylate ion (the -COOH will lose a proton to become -COO- )
The carboxylate ion (-COO-) will form a salt with a positively charged metal ion such as sodium (-COO-Na+)
The clear blue colour of the solution turns opaque red due to the formation of a copper(I) oxide precipitate
Ketones cannot be oxidised and therefore give a negative test when warmed with Fehling’s solution

The copper(II) ions in Fehling’s solution are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to copper(I) ions in the Cu2O precipitate
Tollens’ reagent
Tollens' reagent is an aqueous alkaline solution of silver nitrate in excess ammonia solution
Tollen’s reagent is also called ammoniacal silver nitrate solution
When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Ag+ ions are reduced to Ag atoms
In the alkaline conditions, the carboxylic acid will become a carboxylate ion and form a salt
The Ag atoms form a silver ‘mirror’ on the inside of the tube
Ketones cannot be oxidised and therefore give a negative test when warmed with Tollens’ reagent

The Ag+ ions in Tollens’ reagent are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to silver atoms

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
